|
|
| | Apicycline Basic information |
| Product Name: | Apicycline | | Synonyms: | Apicycline;(-)-N-[[4-(2-Hydroxyethyl)piperazino](carboxy)methyl]tetracycline;1-Piperazineacetic acid, α-[[[4-(dimethylamino)-1,4,4a,5,5a,6,11,12a-octahydro-3,6,10,12,12a-pentahydroxy-6-methyl-1,11-dioxo-2-naphthacenyl]carbonyl]amino]-4-(2-hydroxyethyl)- | | CAS: | 15599-51-6 | | MF: | C30H38N4O11 | | MW: | 630.64 | | EINECS: | | | Product Categories: | | | Mol File: | 15599-51-6.mol | 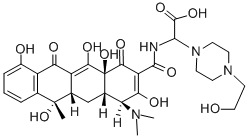 |
| | Apicycline Chemical Properties |
| Melting point | 144.5° (dec) | | alpha | D -123° (c = 0.5 in methanol); D -133° (c = 0.5 in water) |
| | Apicycline Usage And Synthesis |
| Originator | Travert,Baxter | | Manufacturing Process | Glyoxylic acid monohydrate (1.96 g, 20 mrnoles) is dissolved in 200 ml of
methanol, and a solution of 2.60 g (20 mmoles) of N-(2-hydroxyethyl)
piperazine in 50 ml of methanol is slowly added thereto with stirring.
Stirring is maintained for 15 minutes and the medium is cooled to 5°C. There
is then added 10.2 g (20 mmoles) of tetracycline base trihydrate and the
reaction medium is maintained for 20 hours at 5°C under stirring. After that
reaction time, the solution is concentrated up to 50 ml and 0.25 liter of
acetone is added for precipitating a yellowish product which is filtered and
dried to yield N-[(carboxy)(4-β-hydroxyethylpiperazino)methyl] tetracycline
(apicycline).
When tested for antibiotic potency against Bacillus cereus var. mycoides ATCC
9634 (diffusion method), the product is shown to present an antibiotic
potency equivalent to 97% of the tetracycline present therein.
Anhydrous tetracycline base (0.90 g, 2 mmoles) is dissolved in 30 ml of nbutanol
at about 40°C. There is then added thereto under stirring a solution of
0.26 g (2 mmoles) of N-(2-hydroxyethyl)piperazine in 10 ml of butanol and
0.191 g (2 mmoles) of glyoxylic acid monohydrate. A precipitate appears after
a few minutes and the suspension is maintained for six hours under stirring
and at room temperature.
After that reaction time, precipitation is completed by addition of 120 ml of
ether. The yellowish precipitate is filtered and dried to yield N-[(carboxy)(4-β-
hydroxyethylpiperazino)methyl]tetracycline (apicycline). Yellow, amorphous
powder with slight amine odor, MP: 144.5°C (dec). Freely solves in water. [α]D
=-123° (c = 0.5 in methanol); [α]D =-133° (c = 0.5 in water).
The pH value of a 2% aqueous solution of this product is 6.2. When tested for
antibiotic potency against Bacillus cercus var. mycoides ATCC 9634 (diffusion
method), the product is shown to present an antibiotic potency equivalent to
95% of the tetracycline present therein. | | Therapeutic Function | Antibiotic |
| | Apicycline Preparation Products And Raw materials |
|