- Cyclosporin A
-

- $9.00 / 10g
-
2024-04-25
- CAS:59865-13-3
- Min. Order: 10g
- Purity: 99%
- Supply Ability: 10 tons
- Cyclosporin A
-

- $0.00 / 1KG
-
2024-04-23
- CAS:59865-13-3
- Min. Order: 1KG
- Purity: 99
- Supply Ability: 10 tons/per week
- Cyclosporin A
-

- $0.00 / 1kg
-
2024-04-20
- CAS:59865-13-3
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 20tons
|
| Product Name: | Cyclosporin A | | Synonyms: | ol27-400;s7481f1;sandimmun;Cyclospori;1,4,7,10,13,16,19,22,25,28,31-Undecaazacyclotritriacontane, cyclic peptide deriv.;30-Ethyl-33-(1-hydroxy-2-methyl-hex-4-enyl)-1,4,7,10,12,15,19,25,28-nonamethyl-6,9,18,24-tetrakis(2-methylpropyl)-3,21-dipropan-2-yl-1,4,7,10,13,16,19,22,25,28,31-undecazacyclotritriacontane-2,5,8,11,14,17,20,23,26,29,32-undecone;Cipol N;Neoplanta | | CAS: | 59865-13-3 | | MF: | C62H111N11O12 | | MW: | 1202.61 | | EINECS: | 611-907-1 | | Product Categories: | Signalling;Protein Phosphatase;KAYQUINONE;antibiotic;Anti-cancer&immunity;Inhibitors;Intermediates & Fine Chemicals;Peptides;Pharmaceuticals;Active Pharmaceutical Ingredients;Organics;APIs;1 | | Mol File: | 59865-13-3.mol | 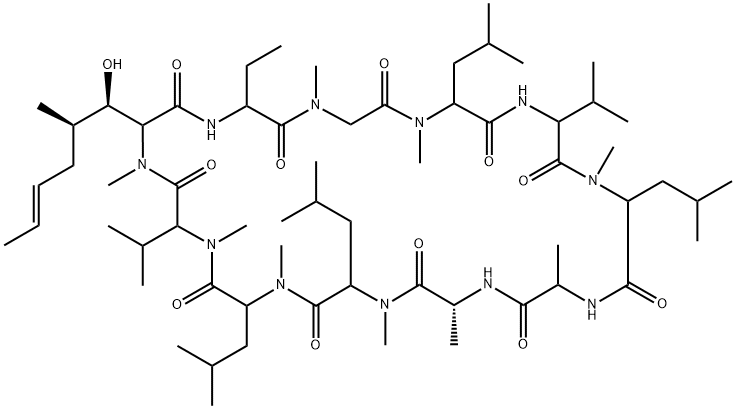 |
| | Cyclosporin A Chemical Properties |
| Melting point | 148-151°C | | Boiling point | 838.63°C (rough estimate) | | alpha | D20 -244° (c = 0.6 in chloroform); D20 -189° (c = 0.5 in methanol) | | density | 0.9913 (rough estimate) | | refractive index | 1.6500 (estimate) | | Fp | 87℃ | | storage temp. | -20°C | | solubility | ethanol: 30 mg/mL | | form | solid | | pka | 13.32±0.70(Predicted) | | color | white | | Water Solubility | Soluble in dimethyl sulfoxide and ethanol. Insoluble in water. | | Merck | 14,2752 | | BRN | 3647785 | | Stability: | Stable for 3 years as supplied. Solutions in DMSO or ethanol may be stored at -20°C for up to 3 months. | | InChIKey | PMATZTZNYRCHOR-CGLBZJNRSA-N | | CAS DataBase Reference | 59865-13-3(CAS DataBase Reference) | | IARC | 1 (Vol. 50, 100A) 2012 | | EPA Substance Registry System | Cyclosporin A (59865-13-3) |
| | Cyclosporin A Usage And Synthesis |
| Immunosuppressants | Cyclosporin A is a lipophilic cyclic peptide compounds separated by certain Hyphomycetes fungi, such as Cylindrocarpon lucidum and Tolypocladium inflatumculture medium. In 1980s, as an immunosuppressant, it has been widely used in the world, is now used for a variety of tissues and organ transplant to prevent rejection reaction and to treat autoimmune diseases.
Cyclosporin A is a strong immunosuppressant, inhibits the onset of the reaction in cell media, reversibly inhibits T cell proliferation, does not inhibit the generation of blood cells, does not affect the function of the phagocyte. As compared with other inhibitors, fewer advantages are induce or aggravate infection, mainly used in clinical kidney, liver, heart, lung, bone marrow transplant anti-rejection, can be combined with adrenal corticosteroids, but not in combination with other immunosuppressive agents. Also used in certain autoimmune diseases (such as systemic lupus erythematosus, lupus nephritis, rheumatoid arthritis, autoimmune hemolytic anemia). Try to uveitis, severe aplastic anemia, refractory idiopathic thrombocytopenic purpura, psoriasis, etc. | | Pharmacological effects | Cyclosporin A can reversibly selectively alter T-lymphocyte function, prevent transcription of lymphokine gene, interfere transfer of original information, inhibit the release of interleukin-2, interferon and other immune factors, affect humoral immunity and immune cells, inhibit killing viability of NK cell; inhibit differentiation and proliferation of lymphocyte in the antigen or mitogen stimulation. But no activity on bone marrow, very small effect on B cells, at therapeutic doses no significant inhibiting effect on granulocyte survival. Oral absorption is slow and incomplete, only 4% to 60%, an average is 30% of the administered dose; relative bioavailability is 20% to 50%. After 2~8h of oral administration reach its peak, with an average of 3.5h. Significant individual differences, such as the relative bioavailability of renal transplant patients is 5% to 89%, liver transplant patients is 8% to 60%. This product is fat-soluble drug, meals and eating food can increase the absorption of fat. Bile deficiency, stagnation, slow gastric emptying, increased gastric peristalsis, pancreatic dysfunction and steatorrhea, all affect absorption of the product.
| | Pharmacokinetics | Oral absorption is irregular and big differences in different individual, bioavailability is 30%, but can be increased with the extended duration of treatment and the increased dose. After liver transplantation, patients with liver disease or disorder of the gastrointestinal absorption may be reduced . Binding rate this product with plasma proteins is up to about 90%, mainly in combination with lipoproteins. Oral peak time is 3 to 4 hours, whole blood concentration is 2 to 9 times of plasma, adult plasma T1/2 is 19 (10-27) hours, while the child is only 7 (7 to 19) hours. This product is metabolized in the liver, by biliary excretion through feces, only 6% by renal excretion, of which about 0.1% is excreted in the primary form.
The above information is edited by the Chemicalbook of Liu Yujie. | | Indications | For prevention of a variety of tissue and organ transplant rejection and treatment of some autoimmune diseases, also used in kidney disease, aplastic anemia, refractory ulcerative colitis. In dermatology, for stubborn and intractable skin diseases, the best effect is severe psoriasis (including vulgaris, pustular, erythrodermic and joint disease); good efficacy on skin disorders including Behcet disease, gangrenous pyoderma, lichen planus, acquired epidermolysis bullosa psychosis; moderate efficacy on disease including alopecia, atopic dermatitis, chronic actinic dermatitis, light class of reticulocyte cell histiocytosis, adult linear IgA bullous dermatitis, pemphigus, bullous pemphigoid (the latter two illness should be in combination with corticosteroids). In addition, Cyclosporine A has effect on systemic lupus erythematosus, dermatomyositis, systemic sclerosis, AIDS, ichthyosis, cutaneous T-cell lymphoma, pityriasis rubra pilaris, generalized missed acrodermatitis, palmoplantar pus blister disease, male pattern baldness, vitiligo, progressive necrotizing xanthogranulomatous, generalized granuloma annulare, lichen myxedema, papular mucin calm disease, hyperthyroidism. | | Drug interactions | 1, In addition to corticosteroids, this product should be avoided in combination with any other immunosuppressive agents.
2, The inactivation of product is mainly in the liver metabolic, any drugs which can affect the liver enzyme activities can affect the metabolism of the product, such as erythromycin, josamycin, doxycycline, ketoconazole, H2 receptor antagonists , calcium antagonists, androgens, oral contraceptives affect activity of cytochrome P-450 liver cell, increase the risk of toxicity of this product.
3, Carbamazepine, phenytoin, phenobarbital, isoniazid, rifampin, et al. can make the plasma concentration of this product decrease.
4, Aminoglycoside antibiotic, trimethoprim-sulfamethoxazole, TMP, amphotericin B, cephalosporins, mechlorethamine, nonsteroidal anti-inflammatory drug, mannitol, furosemide, et al increase nephrotoxicity of cyclosporine .
5, Calcium and increased calcium are disabled when using this product, and cannot be vaccinated.
6, Long-term in combination with glucocorticoid can trigger diabetes, hypertension, ulcer disease and osteoporosis, and can increase the toxicity of this product.
7, Before used Cyclosporin A, other immunosuppressive agents are used, patients have overall decreased immunity and are easy to be infected.
| | Side effects | 1, Kidney toxicity: accounted for about 13%, may have glomerular thrombosis, tubular obstruction, mitochondrial swelling, proteinuria, tubular urine; occasionally hyperuricemia, hyperkalemia, elevated serum creatinine, blood urea nitrogen rise, little or no urine.
2, Liver toxicity: hypoproteinemia, hyperbilirubinemia, serum alkaline phosphatase and lactate dehydrogenase elevated, when the serum concentration of the drug is greater than 200ng/ml most likely to occur, especially in the original hepatitis or liver dysfunction.
3, Nervous system: usually presents exercise-induced spinal cord syndrome, cerebellar ataxia, mental confusion, tremors, abnormal feeling, etc.
4, Gastrointestinal reactions: common anorexia, nausea, and vomiting. Other reactive hypertension, hirsutism. When administered intravenously, rare serious allergic reactions such as chest facial flushing, breathing difficulties, asthma, heart palpitations; once presents the above symptoms, should be discontinued immediately and anti-shock first aid. | | Chemical properties | From-15 ℃ of acetone to obtain white needle crystallization, melting point 148~151 ℃, [α] D20-244 ° (C = 0.6, chloroform). Soluble in methanol, ethanol, acetone, ether or chloroform, slightly soluble in water and saturated hydrocarbons. Acute toxicity LD50 mouse, rat, rabbit (mg/kg): 107, 25, > 10 intravenous injection; 2329,1480,> 1000 oral. | | Production methods | Used Tolypocladium inflatum Gams as production strains. 50L seed medium is added into 75 L tank, inoculated with 5 × 109 spores, Ph = 5.4-4.3, cultured for 72 h, to obtain first order seed liquid. 500L fermentation medium is added into 750 L fermentation tank, inoculated with above first order seed liquid, cultured for 6 days to give second order seed liquid. 3000L fermentation medium is added into 4500L fermentation tank, fermentation for 12 days to give fermented cyclosporine toxicity. An equal volume of ethyl acetate is added into the fermentation broth, organic layer was separated, evaporated under reduced pressure to give the crude product. Melting process, can obtain Cyclosporin A and Cyclosporin C component, wherein Cyclosporin A production is about 150~200 mg/L, Cyclosporin C is about 50~100 mg/L. | | Description | Cyclosporine A is a powerful immunosuppressive drug intended for preventing rejection
of kidney, heart, and lung transplants.
A new era in the development of immunopharmacology began with the discovery of
cyclosporines.
Cyclosporines are produced by mycelial mushrooms Tolypocladium inflatum,
Tricoderma polysporum, and Cylindrocarpon lucidum, which are found in the ground.
Cyclosporine A is the first drug to affect a specific line of protecting cells of the body.
Unlike usual cytotoxics, it suppresses T-cells and acts on all cell lines simultaneously.
Cyclosporine A significantly eases the ‘reception’ of transplants, and increases the possi�bility of treating autoimmune system diseases. | | Chemical Properties | White crystalline solid or powder. | | Chemical Properties | White or almost white powder | | Originator | Sandimmune,Sandoz,US,1983 | | Uses | An immunosuppressant that has revolutionized organ transplantation through its use in the prevention of graft rejection.
A group of nonpolar cyclic oligopeptides with immunosupppressant activity. | | Uses | prothrombogenic agent | | Uses | Cyclosporin A is a hydrophobic cyclic peptide isolated from several fungal species including Cylindrocarpon, Fusarium, Trichoderma and Tolypocladium. Cyclosporin A inhibits T-cell activation and has been marketed since 1983 as an immunosuppressant in post-allogeneic organ transplant. Cyclosporin A acts by binding to the protein, cyclophilin (immunophilin), in T-lymphocytes causing inhibition of calcineurin (protein phosphatase 2B). Cyclosporin A reduces transcription of interleukin 2, and inhibits lymphokine production, interleukin release and NO synthesis induced by interleukin 1α, lipopolysaccharides and TNFα. | | Definition | ChEBI: A cyclic nonribosomal peptide of eleven amino acids; an immunosuppressant drug widely used in post-allogeneic organ transplant to reduce the activity of the patient's immune system, and therefore the risk of organ rejection. Also causes reversible inhibiti
n of immunocompetent lymphocytes in the G0- and G1-phase of the cell cycle. | | Indications | | | Manufacturing Process | 10 liters of a nutrient solution (of which each liter contains 30 g of sucrose,
10 g of corn steep, 3 g of NaNO3, 1 g of K2HPO4, 0.5 g of MgSO4·7H2O, 0.5 g
of KCl and 0.01 g of FeSO4·7H2O) are inoculated with 100 cc of a conidia and mycelium suspension of the strain NRRL 5760, and incubation is effected in
700 cc penicillin flasks at 27°C for 11 days.
The mycelium, which has been separated from the culture liquid, is extracted
in a Turrax apparatus by crushing and stirring with 3.5 liters of 90%
methanol, and the crushed mycelium, which is separated from the solvent by
filtering with suction, is again treated twice in the same manner with 90%
methanol. The combined filtrates are concentrated by evaporation in a
vacuum at a bath temperature of 40°C to such an extent that the vapor
mainly consists of water alone. The resulting mixture is extracted six times
with the same volume of ethylene chloride by shaking, whereupon the
combined ethylene chloride solutions are purified by extraction with water and
are concentrated by evaporation in a vacuum at a bath temperature of 40°C.
The resulting residue is chromatographed on 250 g of silica gel (silica gel 60
Merck, grain size 0.063-0.200 mm), using chloroform containing 2% of
methanol as eluant, and is collected in 200 cc fractions. The fractions which
are antibiotically active against Aspergillus niger in the plate diffusion test are
combined, evaporated to dryness as described above, and after dissolving in
methanol are chromatographed on 110 g of Sephadex LH20 with the same
solvent, whereupon those 20 cc fractions showing an antibiotic effect against
Aspergillus niger in the test indicated above, are combined. A test in the thin
layer chromatogram, e.g., with silica gel on Polygram foils and
hexane/acetone (1:1) as eluant, indicates that the residue of the methanol
solution evaporated as described above mainly consists of the two new
antibiotics S 7481/F-1 and S 7481/F-2. These are separated and
simultaneously purified by a further chromatography of the mixture thereof,
using a 1,000-fold amount of silica gel on the above indicated quality and
chloroform contains 2% of methanol. A testing of the eluate fractions having a
volume in milliliters which is half as large as the weight of the silica gel in
grams, in the thin layer chromatogram, indicates that the antibiotic S 7481/F-
1 appears first in the eluate, followed by a mixture of the two antibiotics and
finally by homogeneous S748l/F-2.
Further amounts of the two antibiotics may be obtained from the mixture by
repeating chromatography under the same conditions. | | Brand name | Gengraf (Abbott); Neoral (Novartis); Restasis (Allergan); Sandimmune (Novartis) [Names previously used: Cyclosporin A; Cyclosporin.]. | | Therapeutic Function | Immunosuppressive | | General Description | White prismatic needles (from acetone) or white powder. | | Air & Water Reactions | Slightly water soluble . | | Reactivity Profile | Cyclosporin A is an amide. Amides/imides react with azo and diazo compounds to generate toxic gases. Flammable gases are formed by the reaction of organic amides/imides with strong reducing agents. Amides are very weak bases (weaker than water). Imides are less basic yet and in fact react with strong bases to form salts. That is, they can react as acids. Mixing amides with dehydrating agents such as P2O5 or SOCl2 generates the corresponding nitrile. The combustion of these compounds generates mixed oxides of nitrogen (NOx). | | Health Hazard | SYMPTOMS: Symptoms of exposure to Cyclosporin A include hepatotoxicity, nephrotoxicity, hyperkalemia, hyperuricemia, convulsions, renal dysfunction, tremor, hirsutism, hypertension, gum hyperplasia, cramps, acne, headache, diarrhea, nausea, vomiting, abdominal discomfort, paresthesia, flushing, leukopenia, lymphoma, sinusitis and gynecomastia. In 2% or less of persons exposed, it has caused allergic reactions, anemia, anorexia, confusion, conjunctivitis, edema, fever, brittle fingernails, gastritis, hearing loss, hiccups, hyperglycemia, muscle pain, peptic ulcer, thrombocytopenia and tinnitus. Rare reactions include anxiety, chest pain, constipation, depression, hair breaking hematuria, joint pain, lethargy, mouth sores, myocardial infarction, night sweats, pancreatitis, pruritus, swallowing difficulty, tingling, upper gastrointestinal bleeding, visual disturbance, weakness and weight loss. It has caused kidney and liver damage. An increased susceptibility to infection may occur. Other symptoms include gastrointestinal disturbance, rashes and angioedema. | | Fire Hazard | Flash point data for Cyclosporin A are not available; however, Cyclosporin A is probably combustible. | | Biochem/physiol Actions | Cyclosporin A is a non-polar cyclic oligopeptide produced by the fungus Tolypocladium inflatum. It is a potent immunosuppressive agent, affecting primarily T-lymphocytes. It has been shown to inhibit the functioning of several nuclear proteins involved in T-cell activation at the level of mRNA transcription. It forms a complex with its intracellular receptor cyclophilin, which can then bind to calcineurin, a Ca2+- and calmodulin-dependent protein phosphatase, inhibiting its enzymatic activity. CsA was found to suppress the replication of hepatitis C virus genome in cultured hepatocytes. At concentrations >10 nM, CsA protected isolated hepatocytes against the action of phalloidin. CsA can inhibit IL2 production resulting from T cell activation via Calcineurin inhibition.An extensive list of references has been reported, including a comprehensive review of analytical properties. | | Pharmacology | Cyclosporine has no direct effect on keratinocytes and is not a mitotic inhibitor. Cyclosporine inhibits cytokine release, which results in a decreased recruitment of APCs into the epidermis and decreases immunoreactivity of lesions. Potential long-term side effects preclude cyclosporine’s use in all but very severe and recalcitrant psoriasis. Cyclosporine can be combined with lowdose methotrexate. | | Clinical Use | Immunosuppressant:
Prophylaxis of solid organ transplant rejection
Nephrotic syndrome
Atopic dermatitis
Psoriasis
Rheumatoid arthritis
Ulcerative colitis | | Side effects | Cyclosporine’s main side effects, even at low doses, are hypertension and nephrotoxicity. Age, baseline blood pressure, and baseline creatinine levels are predictors of higher risks of side effects. Glomerular filtration rate (GFR) is a more sensitive test than creatinine for evaluating renal function, and a baseline is recommended in any high-risk patient. Longterm treatment with CSA may induce interstitial fibrosis and glomerular sclerosis, with more pronounced changes directly associated with duration of therapy. It should be administered only by dermatologists experienced in its use. | | Safety Profile | Confirmed carcinogen
producing Hodghn's dlsease. Experimental
reproductive effects. Poison by
intraperitoneal and intravenous routes.
Moderately toxic by ingestion. Human
systemic effects by ingestion: increased body
temperature, cyanosis. Mutation data
reported. When heated to decomposition it
emits toxic fumes of NOx. | | Synthesis | Cyclosporine A, [R-[R*
,R*
-(E)]]-cyclo-(L-alanyl-D-alanyl-N-methyl-L�leucyl-N-methyl-L-leucyl-N-methyl-L-valyl-3-hydroxy-N,4-dimethyl-L-2-amino-6-
octenoyl-L-α-aminobutyryl-N-methylglycyl-N-methyl-L-leucyl-L-valyl-N-methyl-L-leucine)
(31.2.2), is extracted from a cultural liquid of products of the vital activity of the mush�room Tolypocladium inflatum [14–17], and which is also proposed to obtain synthetically. | | Potential Exposure | Cyclosporin A is a fungal metabolite;
an amide immunosuppressant drug used in various
surgeries. | | Drug interactions | Potentially hazardous interactions with other drugs
Increased risk of hyperkalaemia with ACE
inhibitors, angiotensin-II antagonists, potassium�sparing diuretics, potassium salts.
Increased risk of nephrotoxicity with
aminoglycosides, amphotericin, co-trimoxazole,
disopyramide, foscarnet, melphalan, NSAIDs,
polymyxins, quinolones, sulphonamides, thiazide
diuretics, trimethoprim and vancomycin.
Increased ciclosporin levels with acetazolamide,
aciclovir, amiodarone, atazanavir, boceprevir,
carvedilol, chloramphenicol, chloroquine, cimetidine,
danazol, diltiazem, doxycycline, famotidine,
fluconazole, fluoxetine, fluvoxamine, fosamprenavir,
glibenclamide, glipizide, grapefruit juice,
hydroxychloroquine, imatinib, indinavir, itraconazole,
ketoconazole, lercanidipine (concentration of both
drugs increased - avoid), macrolides, micafungin,
miconazole, high-dose methylprednisolone,
metoclopramide, metronidazole, muromonab�CD3, nicardipine, posaconazole, progestogens,
propafenone, ritonavir, saquinavir and telaprevir
(concentration of both drugs increased), tacrolimus,
verapamil and voriconazole.
Decreased ciclosporin levels with barbiturates,
bupropion, carbamazepine, efavirenz, fosphenytoin,
griseofulvin, lanreotide, modafinil, octreotide,
pasireotide, phenytoin, primidone, quinine, red
wine, rifampicin, St John’s wort, sulfadiazine,
IV sulfadimidine, sulfasalazine, sulfinpyrazone,terbinafine, ticlopidine and IV trimethoprim and
possibly by oxcarbazepine.
Aliskiren: concentration of aliskiren increased -
avoid.
Ambrisentan: concentration of ambrisentan
increased.
Antibacterials: increased risk of myopathy with
daptomycin - try to avoid concomitant use.
Anticoagulants: concentration of dabigatran and
edoxaban increased - avoid with dabigatran and
reduce dose of edoxaban.
Antidiabetics: may increase repaglinide
concentration, risk of hypoglycaemia.
Antimuscarinics: avoid with darifenacin.
Antivirals: avoid with simeprevir, increased
simeprevir concentration; when starting co�administration with dasubavir and ombitasvir/
paritaprevir/ritonavir, give one fifth of the total daily
dose of ciclosporin once daily. Monitor ciclosporin
levels and adjust dose and/or dosing frequency as
needed.
Basiliximab: may alter ciclosporin levels.
Bosentan: co-administration of ciclosporin and
bosentan is contraindicated. When ciclosporin
and bosentan are co-administered, initial trough
concentrations of bosentan are 30 times higher
than normal. At steady state, trough levels are 3-4
times higher than normal. Blood concentrations of
ciclosporin decreased by 50%.
Calcium-channel blockers: increased nifedipine
concentration and toxicity; amlodipine may increase
ciclosporin concentration by up to 40%.
Cardiac glycosides: increased digoxin concentration
and toxicity.
Caspofungin: caspofungin concentration increased -
monitor LFTs.
Colchicine: risk of myopathy or rhabdomyolysis;
also increased blood-ciclosporin concentrations and
nephrotoxicity - avoid.
Cytotoxics: increased risk of neurotoxicity
with doxorubicin; concentration of epirubicin,
everolimus and idarubicin increased; reduced
excretion of mitoxantrone; increased toxicity with
methotrexate; seizures have been reported in bone
marrow transplant patients taking busulfan and
cyclophosphamide; use crizotinib with caution;
concentration of etoposide possibly increased
(increased risk of toxicity); possible interaction with
docetaxol.
Eltrombopag: exposure reduced by ciclosporin.
Fidaxomicin: avoid concomitant use.
Lenalidomide: concentration of lenalidomide
increased.
Lipid-lowering agents: absorption reduced by
colesevelam, increased risk of myopathy with statins
(avoid with simvastatin, max dose of atorvastatin
should be 10 mg1
); avoid with rosuvastatin; increased
risk of nephrotoxicity with fenofibrate; bezafibrate
may increase creatinine and reduce ciclosporin levels;
concentration of both drugs may be increased with
ezetimibe.
Mycophenolate mofetil: some studies show that
ciclosporin decreases plasma MPA AUC levels - no
dose change required.
NSAIDs: diclofenac concentration increased -
reduce diclofenac dose.
Omeprazole: may alter ciclosporin concentration.
Orlistat: absorption of ciclosporin possibly reduced.
Prednisolone: increased prednisolone concentration.
Rifaximin: concentration of rifaximin increased.
Sirolimus: increased absorption of sirolimus -
give sirolimus 4 hours after ciclosporin; sirolimus
concentration increased; long term concomitant
administration may be associated with deterioration
in renal function.
Tacrolimus: increased ciclosporin concentration and
toxicity - avoid.
Ursodeoxycholic acid: unpredictably increased
absorption and raised ciclosporin levels in some
patients. | | Carcinogenicity | Cyclosporin A is known to be a human carcinogen based on sufficient evidence of carcinogenicity from studies in humans. | | Metabolism | Ciclosporin is widely distributed throughout the body.
Distribution in the blood is concentration-dependent,
with between 41-58% in erythrocytes and 10-20% in
leucocytes; the remainder is found in plasma, about 90%
protein-bound, mostly to lipoprotein.
Clearance from the blood is biphasic. Ciclosporin is
extensively metabolised in the liver and mainly excreted
in faeces via the bile. About 6% of a dose is reported to be
excreted in the urine, less than 0.1% unchanged. | | storage | -20°C (desiccate) | | Shipping | UN3249 Medicine, solid, toxic, n.o.s., Hazard
Class: 6.1; Labels: 6.1-Poisonous materials. | | Incompatibilities | Amides/imides react with azo and diazo
compounds to generate toxic gases. Flammable gases are
formed by the reaction of organic amides/imides with
strong reducing agents such as hydrideds and active metals.
Amides are very weak bases (weaker than water). Imides
are less basic yet and in fact react with strong bases to
form salts. That is, they can react as acids. Mixing amides
with dehydrating agents such as such as phosphorus pent-
oxide or thionyl chloride generates the corresponding
nitrile. The combustion of these compounds generates
mixed oxides of nitrogen (NOx). | | Waste Disposal | t is inappropriate and possibly
dangerous to the environment to dispose of expired or waste
drugs and pharmaceuticals by flushing them down the toilet
or discarding them to the trash. Household quantities of
expired or waste pharmaceuticals may be mixed with wet
cat litter or coffee grounds, double-bagged in plastic, discard
in trash. Larger quantities shall carefully take into consider-
ation applicable DEA, EPA, and FDA regulations. If possi-
ble return the pharmaceutical to the manufacturer for proper
disposal being careful to properly label and securely package
the material. Alternatively, the waste pharmaceutical shall be
labeled, securely packaged, and transported by a state
licensed medical waste contractor to dispose by burial in a
licensed hazardous or toxic waste landfill or incinerator. | | References | 1) Liu et al. (1993), FK506 and cyclosporin: molecular probes for studying intracellular signal transduction; Trends Pharmacol. Sci., 18 334 |
| | Cyclosporin A Preparation Products And Raw materials |
|