- Saxagliptin
-

- $0.00/ kg
-
2024-01-02
- CAS:361442-04-8
- Min. Order: 1kg
- Purity: 99%, Single impurity<0.1
- Supply Ability: 1 ton
- Saxagliptin
-

- $110.00 / 1g/Bag
-
2023-06-15
- CAS:361442-04-8
- Min. Order: 1g
- Purity: 99%
- Supply Ability: 1kg
- Saxagliptin
-
- $0.00 / 25kg
-
2023-04-04
- CAS:361442-04-8
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 1mt
|
| | Saxagliptin Chemical Properties |
| Melting point | 103 - 107°C | | Boiling point | 548.7±35.0 °C(Predicted) | | density | 1.35 | | Fp | 548.7℃ | | storage temp. | 2-8°C | | solubility | DMSO (Slightly), Methanol (Sparingly) | | pka | 15.12±0.40(Predicted) | | form | Solid | | color | White to Off-White | | Stability: | Temperature Sensitive |
| | Saxagliptin Usage And Synthesis |
| Treatment Drugs of Type 2 Diabetes | Shacketine is a type 2 diabetes drug that can stimulate the pancreas to produce more insulin after the meal. It was reached by the cooperation of AstraZeneca and Bristol-Myers Squibb Company and belongs to DPP-IV inhibitor. It plays the role through inhibiting GLP-l degradation. GLP-I is the hormones naturally produced in the intestine after taking food. It can regulate the secretion of insulin and strengthen the utilization of glucose in the peripheral tissues. The single medication of Shacketine can improve blood glucose control and the combined medication of Shacketine with metformin, sulfonylurea and thiazolidinediones can enhance curative effect. It leads to low risk of hypoglycemia and its adverse reactions are similar to placebo, showing better tolerance.
On July 31, 2009, Shakleitine tablets (Onglyza), a new drug of type 2 diabetes, jointly researched and developed AstraZeneca and Bristol-Myers Squibb was approved by the US FDA. It can be taken once a day to treat type 2 diabetes combined with controlling Diet and exercise. The most common side effects are upper respiratory tract infections, urinary tract infections and headaches. And other side effects include allergic reactions, such as rash and urticaria. | | Market and Risk | Saxagliptin (rINN), previously identified as BMS-477118, is an oral hypoglycemic (anti-diabetic drug) of the dipeptidyl peptidase-4 (DPP-4) inhibitor class of drugs. Early development was solely by Bristol-Myers Squibb; in 2007 AstraZeneca joined with Bristol-Myers Squibb to co-develop the final compound and collaborate on the marketing of the drug. In June 2008, it was announced that Onglyza would be the trade name under which saxagliptin will be marketed.
In April 2016, the U.S. FDA added a warning about increased risk of heart failure.This was based on data in an article that concluded "DPP-4 inhibition with saxagliptin did not increase or decrease the rate of ischemic events, though the rate of hospitalization for heart failure was increased. Although saxagliptin improves glycemic control, other approaches are necessary to reduce cardiovascular risk in patients with diabetes."
Saxagliptin is used as monotherapy or in combination with other drugs for the treatment of type 2 diabetes. There is no evidence to decrease the risk of heart attacks or strokes.It increases the risk of hospitalization for heart failure by about 27%. Like other DPP-4 inhibitors, it has relatively modest HbA1c lowering ability, is associated with a relatively modest risk of hypoglycemia, and does not cause weight gain. | | Synthesis Method | First synthesize the precursor 109 from 1-adamantanecarboxylic acid and then synthesize the precursor 113 from Boc-L pyroglutamic acid ethyl ester. Finally synthesize Shakleitine from 109 and 113.
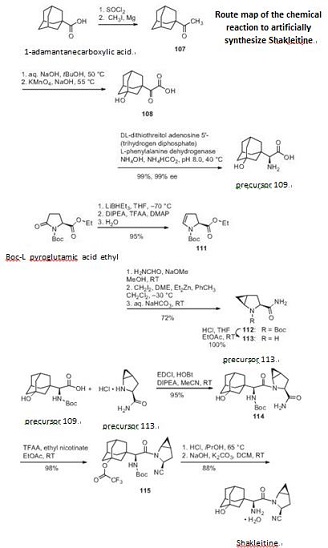
Figure 1 the chemical reaction of synthesizing Shakleitine. | | Chemical Properties | White Solid
 | | Uses | Saxagliptin is a potent and selective reversible inhibitor of dipeptidyl peptidase-4, which is being developed for the treatment of type 2 diabetes. It is absorbed rapidly after oral administration and has a pharmacokinetic profile compatible with once daily dosing. | | Uses | Saxagliptin is a potent and selective reversible inhibitor of dipeptidyl peptidase-4, which is being developed for the treatment of type 2 diabetes. It is absorbed rapidly after oral administration an
d has a pharmacokinetic profile compatible with once daily dosing. | | Definition | ChEBI: A monocarboxylic acid amide obtained by formal condensation of the carboxy group of (2S)-amino(3-hydroxyadamantan-1-yl)acetic acid with the amino group of (1S,3S,5S)-2-azabicyclo[3.1.0]hex
ne-3-carbonitrile. Used in its monohydrate form for the treatment of Type II diabetes. | | Clinical Use | Saxagliptin, previously identified as BMS-477118, is an oral
hypoglycemic of the dipeptidyl peptidase-4 (DPP-4) inhibitor class
developed by Bristol-Myers Squibb for the treatment of type 2 diabetes.
DPP-IV is the primary enzyme responsible for degradation
of incretins, such as glucagon-like peptide-1 (GLP-1), which is a
hormone responsible for the glucose-dependent stimulation of
insulin in humans. Inhibitors of DPP-IV serve as effective glucose
regulators by increasing the endogenous concentration of GLP-1. | | Synthesis | The initial discovery route to saxagliptin was a 15-step, convergent synthesis focused on the production and use of compounds 109
and 113 (Schemes a and b). While the strategy of early
drug delivery involved rapid synthesis to support preclinical
activities and Phase I clinical trials, as saxagliptin entered Phase
II, a greater emphasis was placed on defining and demonstrating
a commercially viable synthetic process. Scheme a describes a
more expedient route to the preparation of adamantylamino acid
109. Commercially available 1-adamantoic acid (106) was first
converted to the corresponding acid halide through the use of
thionyl chloride prior to a Grignard addition reaction utilizing
iodomethane and magnesium metal to furnish ketone 107. This
ketone was then subjected to oxidizing conditions involving potassium
permanganate to provide the hydroxylated ketoacid 108.
The amino acid 109 was furnished through the use of phenylalanine
dehydrogenase in near-quantitative yield in 99% enantioselectivity.
The synthesis of 113 began with commercially available ethyl
N-tert-butoxycarbonylpyroglutamate (110) (Scheme b). Selective
reduction of the amide carbonyl within 110 through the use
of lithium triethylborohydride followed by acylation and baseinduced
elimination of the resulting aminal and careful hydrolysis gave rise to dihydropyrrole 111 with full retention of stereochemical
configuration in 95% yield. Amidation followed by Simmons¨C
Smith cyclopropanation employing methylene iodide converted
111 to the cyclopropanated product 112, which was then converted
to the key coupling partner 113.
The core of saxagliptin was formed by the amide coupling of
amino acid 109 and methanoprolinamide 113 to give amide 114
in 95% yield (Scheme c). Subsequent dehydration of the primary
amide 114 using trifluoroacetic acid anhydride and ethyl nicotinate
gave nitrile 115 in 98% yield. Removal of both the alcohol
and amine protecting groups with HCl afforded saxagliptin (XVIII)
in 88% yield.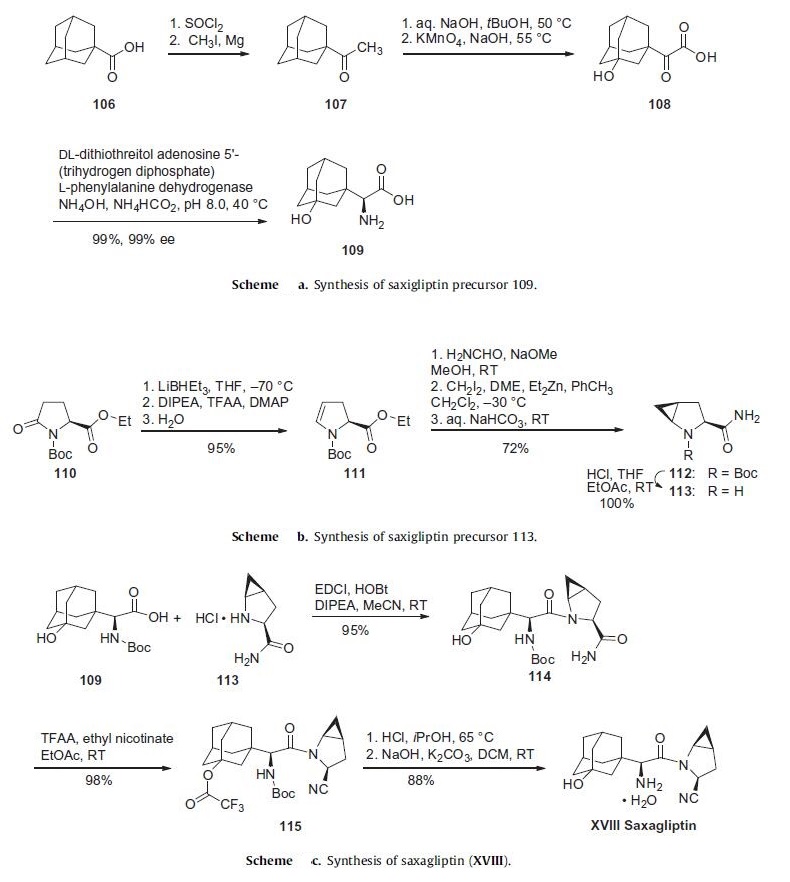
| | Metabolism | Metabolism is mainly by cytochrome P450 3A4/5.
The major metabolite of saxagliptin is also a selective,
reversible, competitive DPP 4 inhibitor, half as potent as
saxagliptin.
Saxagliptin and 5-hydroxy saxagliptin are excreted in
the urine; there may be some active renal excretion of
unchanged saxagliptin. There is also some elimination via
the faeces. |
| | Saxagliptin Preparation Products And Raw materials |
|