- Coumalic acid
-
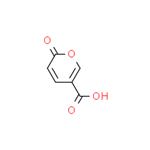
- $26.00 / 25g
-
2025-12-02
- CAS:500-05-0
- Min. Order: 25g
- Purity: 0.97
- Supply Ability: 25kg
- Coumalic acid
-
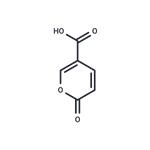
- $29.00 / 1mL
-
2025-12-02
- CAS:500-05-0
- Min. Order:
- Purity: 98.53%
- Supply Ability: 10g
- Coumalic acid
-
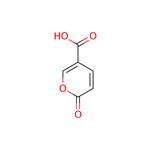
- $1250.00 / 1Kg
-
2025-05-24
- CAS:500-05-0
- Min. Order: 0.100Kg
- Purity: 99 %
- Supply Ability: 500 Kg
|
| | Coumalic acid Basic information |
| Product Name: | Coumalic acid | | Synonyms: | 2-Oxo-2H-pyran-5-carboxylic acid, 2-Pyrone-5-carboxylic acid;6-Oxopyran-3-carboxylic acid;a-Pyrone-5-carboxylic acid;α-Pyrone-5-carboxylic acid;Coumalic acid, 5-Carboxy-2-pyrone;2H-Pyran-5-carboxylic acid, 2-oxo;CouMalic acid 97%;CouMalic Acid, 97.0%(GC&T | | CAS: | 500-05-0 | | MF: | C6H4O4 | | MW: | 140.09 | | EINECS: | 207-899-0 | | Product Categories: | sy | | Mol File: | 500-05-0.mol | 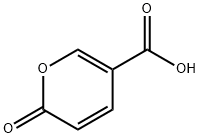 |
| | Coumalic acid Chemical Properties |
| Hazard Codes | Xi | | Safety Statements | 22-24/25 | | WGK Germany | 3 | | TSCA | Yes | | HS Code | 29322090 |
| | Coumalic acid Usage And Synthesis |
| Chemical Properties | pale yellow to pale brown powder | | Uses | Coumalic acid undergoes thermal reaction with 1,3-butadiene to yield dimethyl tricycle[3.2.1.02,7]oct-3-ene-2,4-dicarboxylate. | | Definition | ChEBI: Coumalic acid is a pyranone. | | General Description | Decarboxylates to α-pyrone, a Diels-Alder diene. | | Synthesis | The general procedure for the synthesis of coumaric acid from DL-malic acid was as follows: fuming sulfuric acid (287 g) was slowly added dropwise over a period of 2 hours to a suspension of DL-malic acid (200 g) dissolved in concentrated sulfuric acid (313 g) at 75 °C. After the dropwise addition was completed, the reaction mixture was continued to be stirred at the same temperature for 4 hours. Upon completion of the reaction, the mixture was cooled to room temperature and subsequently poured slowly into ice water over a period of 1 hour. After stirring for 15 minutes, the mixture was left to stand overnight. On the following day, the mixture was cooled to below 10°C and the coumaric acid crude product was isolated by filtration. After washing and drying, coumaric acid was obtained (71 g, 95% purity, 65% yield). | | Purification Methods | The acid crystallises from MeOH. The methyl ester has m 73-74o (from pet ether) and b 178-180o/60 mm. [Beilstein 18/8 V 120.] | | References | [1] Liebigs Annalen der Chemie, 1990, p. 233 - 238
[2] Journal of Heterocyclic Chemistry, 2008, vol. 45, # 1, p. 229 - 234
[3] Patent: WO2004/792, 2003, A1. Location in patent: Page 15
[4] Organic Process Research and Development, 2003, vol. 7, # 1, p. 74 - 81
[5] Patent: CN103788039, 2016, B. Location in patent: Paragraph 0039; 0041-0044 |
| | Coumalic acid Preparation Products And Raw materials |
|