- Latamoxef USP/EP/BP
-
- $1.10 / 1g
-
2021-07-01
- CAS:64952-97-2
- Min. Order: 1g
- Purity: 99.9%
- Supply Ability: 100 Tons Min
- Latamoxef
-

- $0.00 / 1Kg
-
2020-05-25
- CAS:64952-97-2
- Min. Order: 1KG
- Purity: 99.0%+
- Supply Ability: 300 MT
- Latamoxef
-

- $3.00 / 3KG
-
2019-08-08
- CAS:64952-97-2
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: 100kg
|
| | Latamoxef Basic information |
| Product Name: | Latamoxef | | Synonyms: | 5-Oxa-1-azabicyclo(4.2.0)oct-2-ene-2-carboxylic acid, 7-((carboxy(4-hydroxyphenyl)acetyl) amino)-7-methoxy-3-(((1-methyl-1H-tetrazol-5-yl)thio)methyl)-8-oxo-;5-Oxa-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid, 7-[[(2R)-2-carboxy-2-(4-hydroxyphenyl)acetyl]amino]-7-methoxy-3-[[(1-methyl-1H-tetrazol-5-yl)thio]methyl]-8-oxo-, (6R,7R)-;LMOX;C07231;5-oxa-1-azabicyclo(4.2.0)oct-2-ene-2-carboxylicacid,7-((carboxy(4-hydroxyphen;lamoxactam;latamoxef;Latamoxef
7-((Carboxy(4-hydroxyphenyl)acetyl)amino)-7-methoxy-(3-((1-methyl-1H-tetrazol-5-yl)thio)methyl)-8-oxo-5-oxa-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid | | CAS: | 64952-97-2 | | MF: | C20H20N6O9S | | MW: | 520.47 | | EINECS: | 265-287-9 | | Product Categories: | | | Mol File: | 64952-97-2.mol | 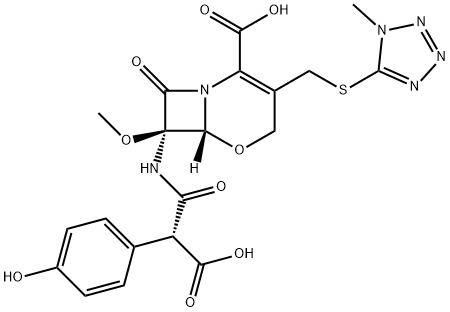 |
| | Latamoxef Chemical Properties |
| Melting point | 117-122° (dec) | | alpha | D25 -15.3 ±2.6° (c = 0.216 in methanol) | | density | 1.77±0.1 g/cm3(Predicted) | | storage temp. | Sealed in dry,2-8°C | | pka | 3.25±0.40(Predicted) |
| WGK Germany | 3 | | RTECS | RN6824500 |
| | Latamoxef Usage And Synthesis |
| Originator | Moxam,Lilly,US,1981 | | Uses | Latamoxef is an oxacephem antibiotic usually grouped with the cephalosporins. An anti-bacterial agent. | | Uses | Anti-infective. | | Definition | ChEBI: A broad-spectrum oxacephem antibiotic in which the oxazine ring is substituted with a tetrazolylthiomethyl group and the azetidinone ring carries methoxy and 2-carboxy-2-(4-hydroxyphenyl)acetamido substituents. | | Manufacturing Process | To a stirred suspension of p-(p-methoxybenzyloxy)-phenylmalonic acid (125
mg) in methylene chloride (3 ml) are added triethylamine (55 l) and oxalyl
chloride (26 l) at -15°C, and the suspension is stirred for 40 minutes at 0°C.
The mixture is added to a solution of diphenylmethyl 7β-amino-7α-methoxy-3-
(1-methyltetrazol-5-yl)thiomethyl-1-oxadethia-3-cephem-4-carboxylate (100
mg) in methylene chloride (3 ml) and pyridine (63 l), and the mixture is
stirred for 30 minutes at 0°C. The reaction mixture is diluted with ethyl
acetate, washed with aqueous 2N-hydrochloric acid and water, dried over
sodium sulfate, and concentrated to give crude product (212 mg), which is
chromatographed on silica gel (20 g) and eluted with a mixture of ethyl
acetate and acetic acid (99:1) to give diphenylmethyl-7β-[α-p-(p�methoxybenzyloxy)phenyl-α-carboxyacetamido]-7α-methoxy-3-(1-
methyltetrazol-5yl)thiomethyl-1-oxadethia-3-cephem-4-carboxylate as foam
(71 mg). Yield: 45%.
To a solution of diphenylmethyl-7β-[α-p-(p-methoxybenzyl)-oxy-phenyl-α-p�methoxybenzyl-oxycarbonil-acetamido]-7α-methoxy-3-(1-methyltetrazol-5-
yl)thiomethyl-1-oxadethia-3-cephem-4-carboxylate (1.20 g) in methylene
chloride (24 ml) are added anisole (2.4 ml) and a solution of aluminum
chloride (2.58 g) in nitromethane (12 ml) at 0°C under nitrogen. After stirring
for 15 minutes at 0°C, the mixture is poured into cold 5% sodium hydrogen
carbonate aqueous solution (100 ml) and filtered to remove the formed
precipitate. The filtate is washed twice with methylene chloride (2 x 100 ml),
acidified with 2N-hydrochloric acid to pH 2.60, and poured in a column of high
porous polymer HP-20 (60 ml) sold by Mitsubishi Chemical Industries Ltd. The
column is washed with water (300 ml) and eluted with methanol. The eluate
is concentrated under reduced pressure at room temperature. The residue is
dissolved in methanol, treated with active carbon, and concentrated under
reduced pressure to give 7β(α-p-hydroxyphenyl-α-carboxyacetamido)-7β-
methoxy-3-(1-methyl-tetrazol-5-yl)thiomethyl1-oxadethia-3-cephem-4-
carboxylic acid as powder (595 mg) decomposing at 125°C to 132°C. Yield:
88.5%.
To a solution of 7β(α-p-hydroxyphenyl-α-carboxyacetamido)-7α-methoxy-3-(1-
methyl-tetrazol-5-yl)thiomethyl1-oxadethia-3-cephem-4-carboxylic acid (359
mg) in methanol (7 ml) is added a solution of sodium 2-ethylhexanoate in
methanol (2 mols/liter; 1.73 ml) at room temperature. After stirring for 10
minutes, the reaction mixture is diluted with ethyl acetate, stirred for 5
minutes, and filtered to collect separated solid, which is washed with ethyl
acetate, and dried to give disodium salt of 7β(α-p-hydroxyphenyl-α-
carboxyacetamido)-7α-methoxy-3-(1-methyl-tetrazol-5-yl)thiomethyl1-
oxadethia-3-cephem-4-carboxylic acid (342 mg). Yield: 888%. Colorless
powder. MP decomposition from 170°C. | | Brand name | Moxam (Lilly);Baxal;Betalactam;Festamoxin;Latoxacet;Mactam;Moxacef;Moxatres;Priollat;Sectam;Shiomalin;Shiomarin. | | Therapeutic Function | Antiinfective | | World Health Organization (WHO) | Latamoxef, a cefamycin antibiotic, was introduced in 1982 for the treatment of serious infections. Its use has subsequently been associated with
reports of clinically important haemorrhage, sometimes fatal, and in some
countries routine co-administration of vitamin K is advised to minimize this risk. | | Antimicrobial activity | Moxalactam. A semisynthetic 7-methoxyoxacephem, supplied
as the disodium salt.It is generally slightly
less active than cefotaxime, especially against Staph. aureus,
but unlike other group 4 cephalosporins it exhibits fairly good
activity against B. fragilis. Other Bacteroides spp. are generally
less susceptible. The 7-methoxy substitution, also found in
cephamycins such as cefoxitin, confers resistance to hydrolysis
by a wide range of β-lactamases including those of Staph.
aureus, various enterobacteria and B. fragilis. Resistance, predominantly
in Enterobacter spp., Ps. aeruginosa and Ser. marcescens
due to induction of chromosomal enzymes, has
been found in vitro and in some patients.
A 500 mg intramuscular injection achieves a serum concentration
of 12–22 mg/L after 1.2 h. Infusion of 1 g over
30 min results in a concentration of 60 mg/L. The plasma
half-life is c. 2 h and plasma protein binding 40–50%. There is reasonably good penetration into serous fluids, the concentration
in ascitic fluid reaching 75% and in pleural fluid 50%
of the concentration simultaneously present in the serum.
Levels of 5–35 mg/L have been obtained in inflamed meninges.
Sputum levels are of the order of 2 mg/L following 1 g of
the drug intravenously.
Renal elimination accounts for 90% of the clearance, but
significant concentrations are found in the feces. Excretion is
depressed in renal failure. Hemodialysis removes 48–51% of
the drug in 4 h; peritoneal dialysis has little or no effect.
Increased bleeding and decreases in platelet function associated
with the methylthiotetrazole side chain are sufficiently
common to have been cited as reasons for restricting use of
the agent. Use is contraindicated in patients on anticoagulant
therapy. Uses are similar to those of group 4 cephalosporins.
It is generally less successful in the treatment of infections due
to Gram-positive organisms. |
| | Latamoxef Preparation Products And Raw materials |
|