| Company Name: |
BOC Sciences
|
| Tel: |
1-631-485-4226; 16314854226 |
| Email: |
info@bocsci.com |
| Products Intro: |
Product Name:Piroheptine
CAS:16378-21-5
Purity:> 95% Remarks:Piroheptine
|
| Company Name: |
BOC Sciences
|
| Tel: |
16314854226 |
| Email: |
info@bocsci.com |
| Products Intro: |
Product Name:Piroheptine
CAS:16378-21-5
Purity:>95%
|
| Company Name: |
CLEARSYNTH LABS LTD.
|
| Tel: |
+91-22-45045900 |
| Email: |
sales@clearsynth.com |
| Products Intro: |
Product Name:Piroheptine
CAS:16378-21-5
|
|
| | PIROHEPTINE Basic information |
| Product Name: | PIROHEPTINE | | Synonyms: | PIROHEPTINE;1-Ethyl-3-(10,11-dihydro-5H-dibenzo[a,d]cyclohepten-5-ylidene)-2-methylpyrrolidine;3-(10,11-Dihydro-5H-dibenzo[a,d]cyclohepten-5-ylidene)-1-ethyl-2-methylpyrrolidine;3-(10,11-Dihydro-5H-dibenzo[a,d][7]annulen-5-ylidene)-1-ethyl-2-methylpyrrolidine;Pyrrolidine, 3-(10,11-dihydro-5H-dibenzo[a,d]cyclohepten-5-ylidene)-1-ethyl-2-methyl- | | CAS: | 16378-21-5 | | MF: | C22H25N | | MW: | 303.44 | | EINECS: | | | Product Categories: | | | Mol File: | 16378-21-5.mol | 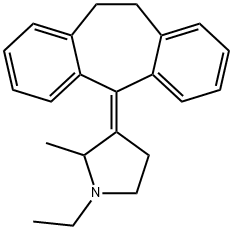 |
| | PIROHEPTINE Chemical Properties |
| Boiling point | bp4 167° | | density | 0.9930 (rough estimate) | | refractive index | 1.4900 (estimate) | | pka | 8.55±0.40(Predicted) |
| | PIROHEPTINE Usage And Synthesis |
| Originator | Trimol,Fujisawa ,Japan ,1974 | | Definition | ChEBI: Piroheptine is a carbotricyclic compound and a N-alkylpyrrolidine. It has a role as an antiparkinson drug and a muscarinic antagonist. It is a conjugate base of a piroheptine(1+). | | Manufacturing Process | (1) To 3.8 g of 2-methyl-3-(10,11-dihydro-5H-dibenzo[a,d]cycloheptene-5ylidene)-1-pyrroline, there were added 8 g of ethyl iodide. This mixture was placed into a closed vessel and heated at 80°C in a water-bath for one hour. After completing the reaction, the reaction mixture was cooled and the unreacted ethyl iodide was distilled off to yield 5.5 g of 1-ethyl-2methyl-3(10,11-dihydro-5H-dibenzo[a,d]cycloheptene-5-ylidene)-1-pyrrolinium iodide in the form of yellow crystals. These crystals were recrystallized from a mixture of acetone and ether to yield yellow needles of the melting point 223°C.
(2)1-Ethyl-2-methyl-3-(10,11)-dihydro-5H-dibenzo[a,d]cycloheptene-5ylidene)-1-pyrroliniumiodide (4.7 g) was dissolved in 7 cc of methanol. To this solution there were added 1.4 g of sodium boron hydride within about 80 minutes with stirring and stirring of the solution was continued for two hours to complete the reaction. The reaction mixture was acidified with 10% aqueous hydrochloric acid solution and then the methanol was distilled off. The residual solution was alkalized with 20% aqueous sodium hydroxide solution and extracted with ether. The ether layer was dried over magnesium sulfate and the ether was distilled off. The resulting residue was further distilled under reduced pressure to yield 2.0 g of 1-ethyl-2-methyl-3-(10,11)dihydro-5H-dibenzo[a,d]cycloheptene-5-ylidene)pyrrolidine (boiling point 167°C/4 mm Hg.). | | Therapeutic Function | Antiparkinsonian |
| | PIROHEPTINE Preparation Products And Raw materials |
|