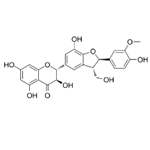
- Silychristin
-
- $0.00 / 25kg
-
2024-03-28
- CAS:33889-69-9
- Min. Order: 25kg
- Purity: 30% 50%(HPLC) 80%(UV)
- Supply Ability: Inquiry
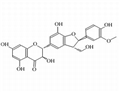
- Silychristin
-
- $0.00 / 5mg
-
2023-02-24
- CAS:33889-69-9
- Min. Order: 5mg
- Purity: ≥98%(HPLC)
- Supply Ability: 10 g
- Silicristin
-

- $1.00 / 1KG
-
2019-07-06
- CAS:33889-69-9
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: Customized
|
| | Silychristin Basic information |
| | Silychristin Chemical Properties |
| Melting point | 174~176℃ | | Boiling point | 782.0±60.0 °C(Predicted) | | density | 1.578±0.06 g/cm3(Predicted) | | storage temp. | 2-8°C | | solubility | Acetone (Slightly), Methanol (Slightly) | | pka | 7.39±0.60(Predicted) | | color | White to Off-White | | Water Solubility | practically insoluble in water | | Stability: | Hygroscopic |
| Hazard Codes | Xn | | Risk Statements | 22 | | Safety Statements | 22-45 | | WGK Germany | 3 |
| | Silychristin Usage And Synthesis |
| Chemical Properties | Silychristin is the second most abundant flavonolignan in the silymarin complex, which is usually produced by the acetone extraction of Silybum marianum (L.) Gaertn. (milk thistle) fruits. Natural silychristin is a mixture of two diastereomers (silychristin A and B, 95:5). Isosilychristin is a silychristin isomer mainly occurring in wild milk thistle. At the same time, 2,3-dehydrosilychristin is a product of its aerial oxidation detectable in silymarin preparations. In contrast, anhydrosilychristin is a dehydrated product obtained by treating silychristin with hydrochloric acid in hot ethanol[1].
| | Uses | Silychristin is a new natural product has been isolated from silymarin, the hepatoprotective extract of milk thistle (Silybum marianum) fruits. Silychristin has been shown to be an antihepatotoxic flavonolignan. | | Definition | ChEBI: A flavonolignan isolated from Silybum marianum and has been shown to exhibit inhibitory activities against lipoxygenase and prostaglandin synthetase. | | benefits | Like other components of the silymarin complex, silychristin has antioxidant properties. Its potential to scavenge the 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical is nearly 14× higher than that of silybin (considered “the active component of silymarin”) and approximately 1.5× lower than that of its oxidized derivative 2,3-dehydrosilybin. This compound exhibited a higher antioxidant capacity than “traditional” antioxidants.
Silychristin was able to inhibit α-glucosidase, exhibiting a potential in the treatment of diabetes mellitus type II. Furthermore, it increased insulin secretion and decreased glucose content in induced type I diabetic rats and Mesocestoides vogae larvae.
Silychristin also displayed concentration-dependent anti-inflammatory activity and inhibited collagenase much more efficiently than its standard inhibitor 1,10-phenanthroline, thus showing a potential application in cosmeceuticals. In a transdermal study, silychristin penetrated the human skin but did not reach the basolateral side. Both acute cytotoxic and genotoxic doses were higher than 100 μM for blood platelets, peripheral blood mononuclear cells, and alveolar basal epithelial cells. Moreover, at these concentrations, this compound protected mitochondria against spontaneous DNA damage. Moreover, copper and iron chelation was also affected by silychristin, which could have implications for the absorption of these ions in the gastrointestinal tract[1]. | | in vitro |
Silychristin exhibits a strong inhibition of MCT8-mediated T3 uptake with an IC50 of 110 nM in MCT8 overexpressing MDCK1-cells.
It causes no cytotoxic for fibroblasts.
Silychristin (6.5-75 μM; 24 hours) diminishes UVA toxicity and reduces ROS generation, and the protective effect is dose-dependent.
Silychristin (12.5μM, 25μM) reduces the metalloproteinase-1 (MMP-1) level in cells.
| | target | NADPH-oxidase | P450 (e.g. CYP17) | | References | [1] Simona Dobiasová. “Multidrug Resistance Modulation Activity of Silybin Derivatives and Their Anti-inflammatory Potential.” Antioxidants 9 5 (2020).
|
| | Silychristin Preparation Products And Raw materials |
|