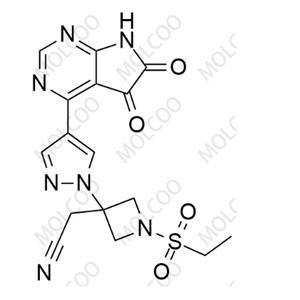
Baricitinib Impurity 92;271-70-5
WhatsAPP: +86 17386083646
E-mail: anna@molcoo.com
Product Number: B026092
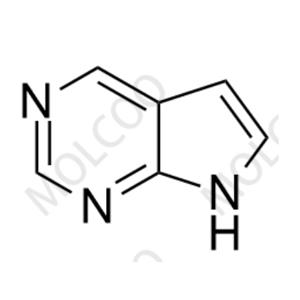
English Name: Baricitinib Impurity 92
English Alias: 7H-pyrrolo[2,3-d]pyrimidine
CAS Number: 271-70-5
Molecular Formula: C₆H₅N₃
Molecular Weight: 119.12
As an impurity reference standard for baricitinib, this compound has the following advantages:
Simple and stable structure, which can be used as a basic raw material to analyze the side reaction mechanism of pyrrolopyrimidine ring construction in baricitinib synthesis and optimize cyclization reaction conditions;
As a standard for heterocyclic compounds, it provides a reference for the detection of nitrogen-containing heterocyclic impurities in drugs, improving the quantitative accuracy of methods such as HPLC;
Helps study the impact of the pyrrolopyrimidine parent nucleus structure on drug stability and activity, providing a theoretical basis for impurity control strategies.
Quality Control: Used as an impurity reference standard for HPLC detection of baricitinib APIs and formulations to verify whether the content of this impurity meets pharmacopoeia limits;
Drug Development: In generic drug research, comparing the impurity profile of the original drug to ensure quality consistency between the generic and original drug;
Process Optimization: Guiding the optimization of parameters such as cyclization reaction temperature and catalyst selection in the synthesis route by analyzing the formation path of this impurity to reduce impurity generation.
Baricitinib is a JAK inhibitor used in the treatment of rheumatoid arthritis and other autoimmune diseases. If the cyclization reaction is incomplete or out of control during synthesis, heterocyclic impurities such as 7H-pyrrolo[2,3-d]pyrimidine are easily generated from the pyrrolopyrimidine ring in its molecular structure. Since heterocyclic compounds may affect the safety and effectiveness of drugs, research on this impurity is an important part of baricitinib quality control.
Current research focuses on:
Detection Methods: Establishing highly sensitive detection methods for this impurity using HPLC-UV or LC-MS technology to achieve trace analysis;
Synthesis Process: Developing high-purity impurity synthesis routes to reduce by-product generation by optimizing the solvent system and reaction time of cyclization reactions;
Toxicological Evaluation: Studying the potential genotoxicity of this heterocyclic structure through in vitro cell experiments to provide data support for establishing safe limits;
Stability Studies: Investigating the degradation behavior of this impurity under high temperature and high humidity conditions to evaluate its impact on baricitinib formulation stability.
We can also customize related analogues and modified peptides including HPLC, MS, 1H-NMR, MS, HPLC, IR, UV, COA, MSDS.
This product is intended for laboratory use only!
WhatsAPP: +86 17386083646
E-mail: anna@molcoo.com










 China
China