Pomalidomide Impurity 54;7607-72-9
WhatsAPP: +86 17386083646
E-mail: anna@molcoo.com
Product Number: P059054
English Name: Pomalidomide Impurity 54
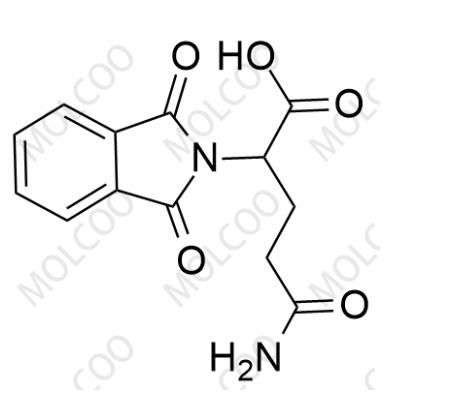
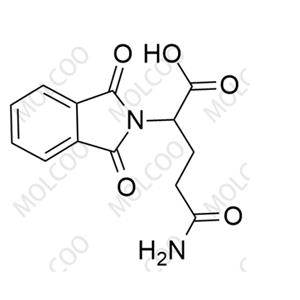
English Alias: 5-amino-2-(1,3-dioxoisoindolin-2-yl)-5-oxopentanoic acid
CAS Number: 7607-72-9
Molecular Formula: C₁₃H₁₂N₂O₅
Molecular Weight: 276.24
As an impurity reference standard for pomalidomide, this compound has significant advantages:
With a clear structure and strong stability, it can be used to deeply study the side reaction mechanisms of reactions such as isoindoline ring connection and amino group introduction during pomalidomide synthesis, optimize the production process, and reduce impurity generation.
As an impurity standard with amino, carboxyl, and isoindolinedione structures, it can provide an accurate reference for the detection of similar complex - structured impurities in drugs, significantly improving the quantitative accuracy of analytical methods such as HPLC and LC - MS.
It helps to explore the impact of the impurity structure on the stability and toxicological properties of drugs, providing an important basis for the scientific formulation of impurity control strategies.
Drug Development: During the research and development of pomalidomide and its formulations, it is used as an impurity reference standard for identification and quantitative analysis, determining the impurity profile of drugs and evaluating the purity of APIs and formulations.
Quality Control: As a standard substance, it is used to verify the sensitivity and specificity of detection methods such as HPLC and LC - MS, ensuring that the content of this impurity during production meets pharmacopoeia and relevant regulatory requirements.
Stability Studies: Investigating the degradation behavior of this impurity under different environmental conditions (light, high temperature, high humidity), evaluating its impact on the stability of pomalidomide formulations, and providing data support for determining drug storage conditions and shelf life.
Pomalidomide is an immunomodulatory drug used in the treatment of hematological diseases such as multiple myeloma. Due to its complex synthesis process with multiple reaction steps, if there are raw material residues, improper reaction temperature, or catalyst use, impurities such as 5 - amino - 2-(1,3 - dioxoisoindolin - 2 - yl)-5 - oxopentanoic acid are likely to be generated. The presence of such impurities may affect the safety, effectiveness, and bioavailability of the drug. Therefore, the research and control of pomalidomide impurities are key links in ensuring drug quality.
Currently, research on Pomalidomide Impurity 54 mainly focuses on the following aspects:
Detection Technologies: Using advanced technologies such as ultra - high - performance liquid chromatography - tandem mass spectrometry (UPLC - MS/MS) and high - resolution mass spectrometry (HRMS) to develop highly sensitive and selective detection methods for trace detection of this impurity.
Synthesis Processes: Deeply studying the formation pathway of this impurity, and developing synthesis processes that reduce impurity generation by optimizing reaction conditions (such as reaction temperature, time, solvent selection) and raw material ratios.
Toxicological Evaluation: Evaluating the potential toxicity of this impurity through in vitro cytotoxicity experiments and animal models to provide data for formulating reasonable impurity limit standards.
Stability Studies: Systematically studying the stability of this impurity under different environmental factors, analyzing its impact on the quality of pomalidomide formulations, and improving the drug quality control system.
We can also customize related analogues and modified peptides including HPLC, MS, 1H-NMR, MS, HPLC, IR, UV, COA, MSDS.
This product is intended for laboratory use only!
WhatsAPP: +86 17386083646
E-mail: anna@molcoo.com











 China
China