Product Details
| Product Name: Sildenafil EP Impurity D | CAS No.: 1357931-55-5 |
| Min. Order: 10mg | Purity: 99%+ HPLC |
| Supply Ability: 1000 | Release date: 2025/07/31 |
| Molecular formula: C17H20N4O5S |
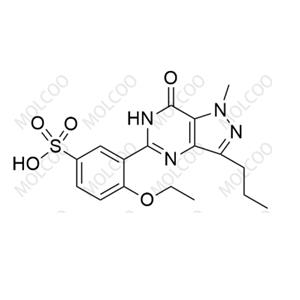
Sildenafil EP Impurity D
WhatsAPP: +86 17386083646
E-mail: anna@molcoo.com.
Product Information
Product Number: S005004
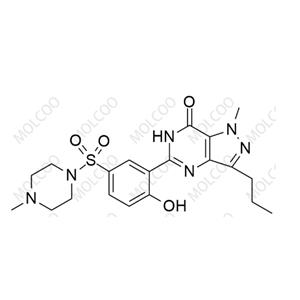
English Name: Sildenafil EP Impurity D
English Alias: 4-ethoxy-3-(1-methyl-7-oxo-3-propyl-6,7-dihydro-1H-pyrazolo[4,3-d]pyrimidin-5-yl)benzenesulfonic acid
CAS Number: 1357931-55-5
Molecular Formula: C₁₇H₂₀N₄O₅S
Molecular Weight: 392.43
Advantages
Well-defined and distinct structure: Contains pyrazolo[4,3-d]pyrimidinone core, ethoxy group, and benzenesulfonic acid moiety, differing from sildenafil by a sulfonic acid (-SO₃H) instead of piperazine on the benzene ring. Significant polarity difference enables accurate identification via HPLC and LC-MS, providing a specific marker for impurity detection;
High stability and traceability: Strong ionization of sulfonic acid ensures stability in aqueous solutions. As a by-product of benzene ring substitution in sildenafil synthesis, it directly reflects competition between sulfonic acid and piperazine substitution, improving process tracing accuracy;
High detection sensitivity: Unique retention behavior of sulfonic acid in reversed-phase chromatography, combined with UV absorption of pyrazolopyrimidine (230-250nm), enables trace analysis via HPLC-UV or LC-MS, compatible with sulfonated heterocyclic compound detection systems.
Applications
Pharmaceutical quality control: Used as an EP reference standard to quantify Sildenafil EP Impurity D in APIs and formulations, ensuring residual sulfonic acid impurities meet pharmacopoeial standards;
Synthesis optimization: Optimizing benzene ring substitution conditions (e.g., reagent selection, pH) by monitoring impurity levels to suppress sulfonation and enhance piperazine substitution selectivity;
Impurity profile compliance: Validating sildenafil’s impurity profile against EP requirements to support regulatory submissions in quality research.
Background Description
Research Status
Analytical method advancement: Developing UPLC-DAD assays with optimized mobile phase pH for baseline separation of impurity and sildenafil, achieving detection limits as low as 0.05 ppm;
Synthetic pathway refinement: Designing selective substitution strategies to minimize sulfonic acid impurity formation via solvent polarity regulation;
Stability studies: Evaluating impurity degradation under varying pH and temperature to guide sildenafil formulation storage conditions;
Pharmacopoeial method validation: Cross-verifying impurity quantification across platforms to ensure compliance with EP standards
Product Information
Product Number: S005004
English Name: Sildenafil EP Impurity D
English Alias: 4-ethoxy-3-(1-methyl-7-oxo-3-propyl-6,7-dihydro-1H-pyrazolo[4,3-d]pyrimidin-5-yl)benzenesulfonic acid
CAS Number: 1357931-55-5
Molecular Formula: C₁₇H₂₀N₄O₅S
Molecular Weight: 392.43
Advantages
Well-defined and distinct structure: Contains pyrazolo[4,3-d]pyrimidinone core, ethoxy group, and benzenesulfonic acid moiety, differing from sildenafil by a sulfonic acid (-SO₃H) instead of piperazine on the benzene ring. Significant polarity difference enables accurate identification via HPLC and LC-MS, providing a specific marker for impurity detection;
High stability and traceability: Strong ionization of sulfonic acid ensures stability in aqueous solutions. As a by-product of benzene ring substitution in sildenafil synthesis, it directly reflects competition between sulfonic acid and piperazine substitution, improving process tracing accuracy;
High detection sensitivity: Unique retention behavior of sulfonic acid in reversed-phase chromatography, combined with UV absorption of pyrazolopyrimidine (230-250nm), enables trace analysis via HPLC-UV or LC-MS, compatible with sulfonated heterocyclic compound detection systems.
Applications
Pharmaceutical quality control: Used as an EP reference standard to quantify Sildenafil EP Impurity D in APIs and formulations, ensuring residual sulfonic acid impurities meet pharmacopoeial standards;
Synthesis optimization: Optimizing benzene ring substitution conditions (e.g., reagent selection, pH) by monitoring impurity levels to suppress sulfonation and enhance piperazine substitution selectivity;
Impurity profile compliance: Validating sildenafil’s impurity profile against EP requirements to support regulatory submissions in quality research.
Background Description
Research Status
Analytical method advancement: Developing UPLC-DAD assays with optimized mobile phase pH for baseline separation of impurity and sildenafil, achieving detection limits as low as 0.05 ppm;
Synthetic pathway refinement: Designing selective substitution strategies to minimize sulfonic acid impurity formation via solvent polarity regulation;
Stability studies: Evaluating impurity degradation under varying pH and temperature to guide sildenafil formulation storage conditions;
Pharmacopoeial method validation: Cross-verifying impurity quantification across platforms to ensure compliance with EP standards
This product is intended for laboratory use only!
WhatsAPP: +86 17386083646
E-mail: anna@molcoo.com
Company Profile Introduction
You may like
Recommended supplier
| Product name | Price | Suppliers | Update time | |
|---|---|---|---|---|
| $0.00/5mg |
VIP1Y
|
Guangzhou Tosun Pharmaceutical Ltd
|
2025-02-18 | |
| $0.00/10mg |
VIP8Y
|
Guangzhou PI PI BIOTECH INC
|
2022-04-22 | |
| $0.00/10mg |
VIP2Y
|
ShenZhen H&D Pharmaceutical Technology Co., LTD
|
2024-05-10 | |
| $9.80/1KG |
VIP6Y
|
Career Henan Chemical Co
|
2020-02-10 |








 China
China