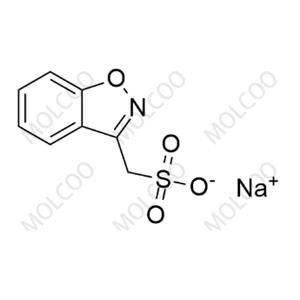
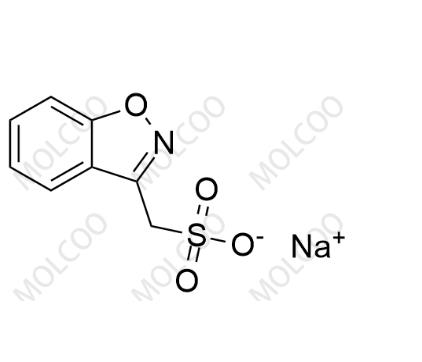
Product Details
| Product Name: Zonisamide USP Related Compound A(Sodium salt) | CAS No.: 73101-64-1 |
| Min. Order: 10mg | Purity: 95%+ |
| Supply Ability: 10000000 | Release date: 2025/07/31 |
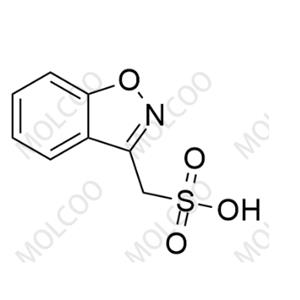
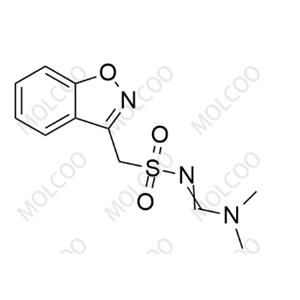
Zonisamide USP Related Compound A(Sodium salt)73101-64-1

Company Profile Introduction
-
You may like
Recommended supplier
| Product name | Price | Suppliers | Update time | |
|---|---|---|---|---|
| $0.00/1000KG |
VIP4Y
|
Zibo Shiji Zhonglian Medical Technology Co., Ltd.
|
2023-02-10 | |
| $0.00/1KG Ton |
VIP4Y
|
ZHUHAI HRD PHARMACEUTICAL CO., LTD.
|
2022-10-09 | |
| $1.00/1KG |
VIP6Y
|
Career Henan Chemical Co
|
2019-12-25 |
INQUIRY









 China
China