N-Ethylmethylamine: sEH Inhibitor Scaffold & Activated Carbon Adsorption
Nov 11,2025
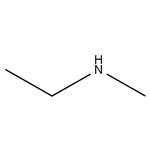
N-Ethylmethylamine is a vital secondary amine that plays a crucial role as an intermediate in both the pharmaceutical and fine chemical industries. N-Ethylmethylamine has been used in the synthesis of N-ethyl-methyl acrylamide via reaction with acryloyl chloride.

Identification of N-ethylmethylamine as a novel scaffold
Fragment-based FBDD) has been developed over the last decade and applied to a number of drug-target proteins. FBDD has been applied to a number of drug-target proteins, and identified several drugs which are currently under clinical study. However, due to the low affinity of fragments for target proteins in FBDD, fragment screening is conducted using biophysical assays such as NMR, SPR, TSA, and X-ray crystallography or biochemical assays with a high-concentration of fragments. Based on the binding mode, N-ethylmethylamine was identified as a promising scaffold that forms hydrogen bonds with the catalytic residues of sEH, Asp335, Tyr383, and Tyr466. This corresponded to a more than 1500-fold increase in inhibitory activity compared to the starting fragment. Co-crystal structures of the hit compounds demonstrate that the binding of N-ethylmethylamine to catalytic residues is similar to that of the starting fragment. We therefore consider crystallographic fragment screening to be appropriate for the identification of weak but promising fragment hits.[1]
Crystallographic fragment screening can detect fragment hits that, although weak, bind specifically to their target. Structural analysis of these hits identified new scaffolds that bind to the catalytic triad of sEH. In particular, N-ethylmethylamine is a promising scaffold for the optimization of compounds to fit the unique catalytic pocket of sEH, which is separated into two sub-pockets via a catalytic triad. The potential of N-ethylmethylamine was demonstrated by the IC50 values of compound 9 (0.51 μM) and 10 (9.0 μM). Structural analysis of compound 9 also revealed a hydroxyl group as a new pharmacophore. Further optimization of N-ethylmethylamine derivatives will help facilitate the identification of novel and potent sEH inhibitors.
Adsorption of N-ethylmethylamine Vapor by Activated Carbon Filters
The application of activated carbon for odor removal has been reported in the patent literature. Human sweat is a source for some of many odorous substances. One of these odorous compounds is N-Ethylmethylamine (EMA). In a previous study, the influence of both pore size distribution and surface chemistry (amount and type of surface groups) on the adsorption of EMA from an aqueous solution was investigated. The results showed that surface chemistry controls the adsorption of EMA at both high and low equilibrium concentrations. At high equilibrium concentrations, the amount adsorbed is directly dependent on the total number of acidic and basic groups on the surface, which indicates the importance of hydrogen bonding. At low concentration, the density of the surface acidic groups enhances the adsorption of ethylmethylamine through acid−base interactions and hydrogen bonding. Porosity plays an important but not predominant role in the strength and extent of the adsorption process. When adsorption of N-Ethylmethylamine from the vapor phase at equilibrium conditions was studied, the mechanism of the process showed that the interactions with the surface acidic groups are energetically more favorable than adsorption in the porous structure. Unfortunately, the kinetics of the process was not evaluated.[2]
Previous results on the adsorption of EMA from an aqueous solution revealed a strong, linear dependence of the amount of N-Ethylmethylamine adsorbed on the quantity of acidic surface groups. The uptake of EMA on the oxidized carbons was higher than that on the initial counterparts. The apparent discrepancy with the data presented here might be related to different experimental conditions, which probably lead to different mechanisms of adsorption. Under the prior assigned experimental conditions, both EMA and the surface acidic groups existed in their ionic forms because the pH of the solution was below the pKa of EMA (pKa ≈ 10.7) and above the pKa of the carboxylic acid groups on the surface. Thus, adsorption of EMA was the result of the direct acid−base reaction between the N-Ethylmethylamine molecules and the surface acidic groups. For the adsorption of EMA from aqueous solution, the influence of surface chemistry on the adsorption of EMA has been clearly established. A higher acidity results in a greater EMA uptake. However, for the adsorption of high quantities of N-Ethylmethylamine vapor from an air stream in a humid environment, this does not hold true. All types of treatment of the initial samples seem to lead to a more hydrophilic behavior of the carbon, resulting in a dramatic loss of adsorption capacity for molecular EMA as a result of blocking of the pore system by water, which is preferentially adsorbed on the surface functional groups. As a result, the liquid- and vapor-phase adsorptions of N-Ethylmethylamine on activated carbons are governed by quite different mechanisms. Carbons developed for the liquid adsorption of EMA are not necessarily suited for vapor-phase applications.
References
[1]Amano, Yasushi et al. “Identification of N-ethylmethylamine as a novel scaffold for inhibitors of soluble epoxide hydrolase by crystallographic fragment screening.” Bioorganic & medicinal chemistry vol. 23,10 (2015): 2310-7. doi:10.1016/j.bmc.2015.03.083
[2] Yehya El-Sayed. (2006). Adsorption of Ethylmethylamine Vapor by Activated Carbon Filters. Industrial & Engineering Chemistry Research, 45 4, 1441–1445.
- Related articles
- Related Qustion
Ethyl 4,4,4-trifluoroacetoacetate is an important intermediate for the synthesis of fluorinated compounds such as pesticides and pharmaceuticals.....
Nov 11,2025Organic Synthesis Intermediate1-Bromooctane undergoes nickel(I) salen-catalyzed reduction, and its production wastewater is treated via crystallization to recover (K,NH4)2SO4 and NH4Br.....
Nov 11,2025Chemical MaterialsN-Ethylmethylamine
624-78-2You may like
N-Ethylmethylamine manufacturers
- N-Ethylmethylamine
-
- $34.00 / 1kg
- 2025-09-25
- CAS:624-78-2
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: g-kg-tons, free sample is available
- N-Ethylmethylamine
-

- $100.00 / 25kg
- 2025-08-08
- CAS:624-78-2
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 50000KG/month
- N-Ethylmethylamine
-

- $0.00 / 25kg
- 2025-07-25
- CAS:624-78-2
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 10000KGS






