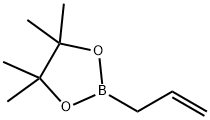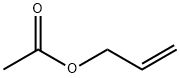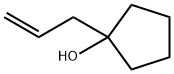
1-allylcyclopentan-1-ol synthesis
- Product Name:1-allylcyclopentan-1-ol
- CAS Number:36399-21-0
- Molecular formula:C8H14O
- Molecular Weight:126.2

120-92-3
427 suppliers
$11.00/25g
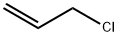
107-05-1
424 suppliers
$16.80/100ML

36399-21-0
3 suppliers
inquiry
Yield:36399-21-0 84%
Reaction Conditions:
with tin(ll) chloride in N,N-dimethyl-formamide at 40; for 48 h;Inert atmosphere;
Steps:
2.3. General procedure for the allylation of aldehydes and ketones with allylic chlorides
General procedure: To a mixture of SnCl2 (1.5 mmol), aldehyde or ketone (1 mmol), and allylic chloride (2 mmol) in DMF (3 mL) was added MCM-41-2N-Pd(II) (60 mg, 0.02 mmol Pd) at the temperature indicated in Table 3 under an argon atmosphere. After being stirred for 48 h, the mixture was filtered and the catalyst was washed with DMF (2 x 10 mL), diethyl ether (2 x 10 mL) and reused in the next run. The filtrate was diluted with 120 mL of a mixed solvent [V(diethyl ether):V(dichloromethane)= 2:1] and washed successively with aqueous 10% HCl solution (2 x 20mL), aqueous NaHCO3 solution (20 mL), and water (3 x 30 mL). The extracts were dried over anhydrous MgSO4. After removal of the solvent under reduced pressure, the residue was purified by flash column chromatography on silica gel [V(hexane):V(ethyl acetate)= 7:1] to afford a colorless oil.
References:
Zhao, Hong;Peng, Jian;Xiao, Ruian;Hao, Wenyan;Cai, Ming-Zhong [Journal of Organometallic Chemistry,2011,vol. 696,# 10,p. 2030 - 2034] Location in patent:experimental part
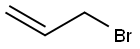
106-95-6
427 suppliers
$10.00/5g

120-92-3
427 suppliers
$11.00/25g

36399-21-0
3 suppliers
inquiry
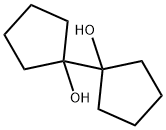
5181-75-9
13 suppliers
$842.37/5G