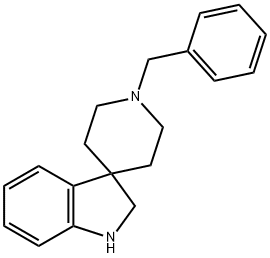
1'-benzylspiro[indoline-3,4'-piperidine] synthesis
- Product Name:1'-benzylspiro[indoline-3,4'-piperidine]
- CAS Number:474538-99-3
- Molecular formula:C19H22N2
- Molecular Weight:278.39
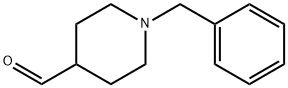
22065-85-6
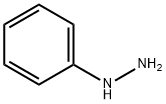
100-63-0
![1'-benzylspiro[indoline-3,4'-piperidine]](/CAS/GIF/474538-99-3.gif)
474538-99-3
1. Preparation of Intermediate Compound 15: To a solution of phenylhydrazine (3.2 mL; 1.1 eq.) dissolved in toluene/acetonitrile (49/1, 50 mL) was slowly added trifluoroacetic acid (16.8 mL; 5 eq.) at room temperature. The reaction mixture was heated to 35 °C. Subsequently, a solution of 1-benzyl-4-piperidinecarboxaldehyde (6 g; 0.03 mol) in toluene/acetonitrile (49/1, 10 mL) was added slowly and dropwise. The reaction mixture was stirred at 35 °C overnight and then cooled to -10 °C. After addition of methanol (7 mL), sodium borohydride (1.7 g; 1.5 eq.) was added in batches. The mixture was continued to stir at room temperature for 1 hour. Upon completion of the reaction, 10% ammonia solution was added and the organic layer was extracted with ethyl acetate, dried over anhydrous magnesium sulfate, filtered and concentrated. The crude product was purified by silica gel column chromatography (75 g SiO2, 35-70 μm; eluent: dichloromethane/methanol 98/2) to afford intermediate compound 15 (3.3 g, 40% yield).

22065-85-6
342 suppliers
$9.00/1g

100-63-0
385 suppliers
$10.00/1g
![1'-benzylspiro[indoline-3,4'-piperidine]](/CAS/GIF/474538-99-3.gif)
474538-99-3
46 suppliers
$56.00/50mg
Yield:474538-99-3 40%
Reaction Conditions:
Stage #1: N-benzyl-4-formylpiperidine;phenylhydrazinewith trifluoroacetic acid in toluene;acetonitrile at 35;
Stage #2: with sodium tetrahydroborate in methanol;toluene;acetonitrile at -10 - 20; for 1 h;
Stage #3: with ammonia;water in methanol;toluene;acetonitrile;
Steps:
A5.a
Example AS; a. Preparation of intermediate compound 15; CF3COOH (16.8 ml; 5 eq) was added at room temperature to a solution of phenylhydrazine (3.2 ml; 1.1 eq) in toluene/CH3CN (49/1) (50 ml). The mixture was heated at 35 °C. A solution of 1-(phenylmethyl)-4-piperidinecarboxaldehyde (6 g; 0.03 mol) in toluene/CH3CN (49/1) (10 ml) was added slowly. The mixture was heated at 35 °C overnight, then cooled down to -10 °C. Methanol (7 ml) was added then NaBH4 (1.7 g; 1.5 eq) was added portionwise. The mixture was stirred at room temperature for 1 hour. NH40H 10 % was added, the mixture was extracted with EtOAc, dried over MgS04, filtered and evaporated. The residue (10 g) was purified by column chromatography over silica gel (75 g Si02 35-70 μm; eluent : 98/2 CH2Cl2/MeOH). Yield: 3.3 g of intermediate compound 15 (40 %)
References:
WO2005/97794,2005,A1 Location in patent:Page/Page column 42