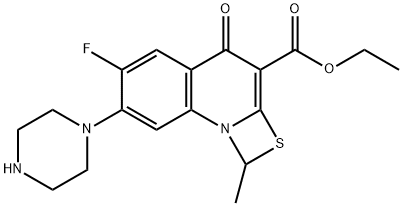
Ethyl 6-fluoro-1-methyl-4-oxo-7-(1-piprazinyl)-4H-[1,3]thiazeto[3,2-a]quinoline-3-carboxylate synthesis
- Product Name:Ethyl 6-fluoro-1-methyl-4-oxo-7-(1-piprazinyl)-4H-[1,3]thiazeto[3,2-a]quinoline-3-carboxylate
- CAS Number:113028-17-4
- Molecular formula:C18H20FN3O3S
- Molecular Weight:377.43

110-85-0
548 suppliers
$5.00/5 g
![Ethyl 6,7-difluoro-1-methyl-4-oxo-4H-[1,3]thiazeto[3,2-a]quinoline-3-carboxylate](/CAS/GIF/113046-72-3.gif)
113046-72-3
132 suppliers
$26.00/100mg
![Ethyl 6-fluoro-1-methyl-4-oxo-7-(1-piprazinyl)-4H-[1,3]thiazeto[3,2-a]quinoline-3-carboxylate](/CAS/GIF/113028-17-4.gif)
113028-17-4
92 suppliers
$83.00/100mg
Yield: 87%
Reaction Conditions:
in dimethyl sulfoxide at 60; for 4 h;
Steps:
1 Preparation of ethyl 6-fluoro-7-piperazine-1-methyl-4-oxo- [1,3] thiazacyclo [3,2-a] quinoline-3-carboxylate
50 g of ethyl 6,7-difluoro-1-methyl-4-oxo-4H- [1,3] thiazine [3,2-a] Dissolve in 5 volumes of DMSO at 60°C, add 40 g of piperazine, and stir at 60°C for 4 hours. The mixture was cooled to room temperature, then 5 volumes of acetonitrile were added and stirring was continued for 4 hours at room temperature. The precipitate was collected by filtration and dried to give 6-fluoro-7-piperazine-1-methyl-4-oxo- [1,3] thiazacyclo [3,2-a] Ethyl acid 52.8g, yield 87%. The yield of 6-fluoro-7-piperazine-1-methyl-4-oxo- [1,3] thiazacyclo [3,2-a] quinoline-3-carboxylic acid B Ester purity 99%.
References:
Zhaoke Pharmaceutical (Hefei) Co., Ltd.;Li Xiaoyi;Dai Xiangrong;Zhou Guanqun CN107383069, 2017, A Location in patent:Paragraph 0061; 0062
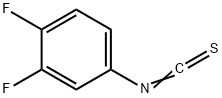
113028-75-4
128 suppliers
$10.00/1g
![Ethyl 6-fluoro-1-methyl-4-oxo-7-(1-piprazinyl)-4H-[1,3]thiazeto[3,2-a]quinoline-3-carboxylate](/CAS/GIF/113028-17-4.gif)
113028-17-4
92 suppliers
$83.00/100mg
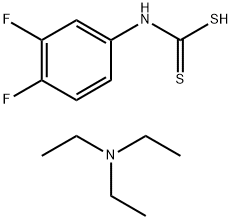
144514-15-8
0 suppliers
inquiry
![Ethyl 6-fluoro-1-methyl-4-oxo-7-(1-piprazinyl)-4H-[1,3]thiazeto[3,2-a]quinoline-3-carboxylate](/CAS/GIF/113028-17-4.gif)
113028-17-4
92 suppliers
$83.00/100mg
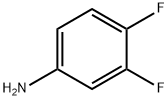
3863-11-4
326 suppliers
$10.00/25 g
![Ethyl 6-fluoro-1-methyl-4-oxo-7-(1-piprazinyl)-4H-[1,3]thiazeto[3,2-a]quinoline-3-carboxylate](/CAS/GIF/113028-17-4.gif)
113028-17-4
92 suppliers
$83.00/100mg
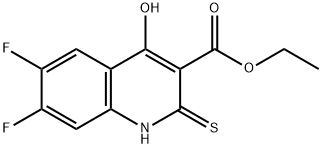
84339-06-0
4 suppliers
inquiry
![Ethyl 6-fluoro-1-methyl-4-oxo-7-(1-piprazinyl)-4H-[1,3]thiazeto[3,2-a]quinoline-3-carboxylate](/CAS/GIF/113028-17-4.gif)
113028-17-4
92 suppliers
$83.00/100mg