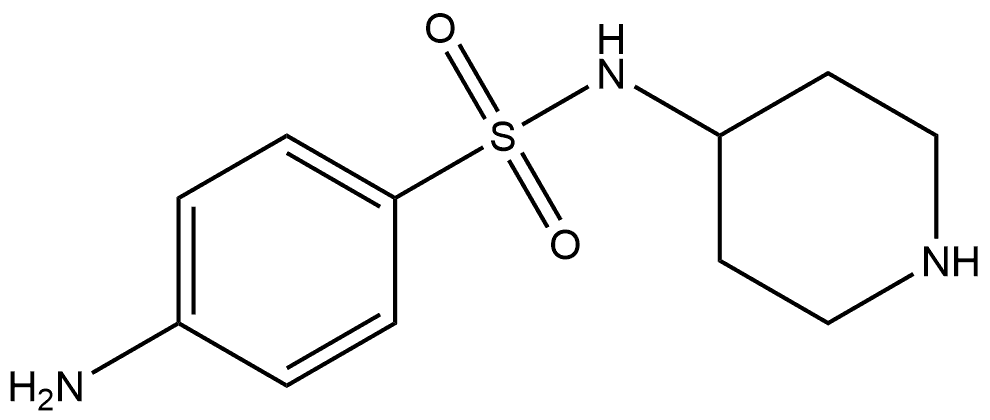
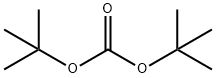
tert-Butyl 4-(4-aminophenylsulfonamido)piperidine-1-carboxylate synthesis
- Product Name:tert-Butyl 4-(4-aminophenylsulfonamido)piperidine-1-carboxylate
- CAS Number:746630-27-3
- Molecular formula:C16H25N3O4S
- Molecular Weight:355.45
Yield:746630-27-3 82%
Reaction Conditions:
with triethylamine in tetrahydrofuran;dichloromethane at 0 - 20;
Steps:
BB.1
First the starting material, 4- (4-ACETYLAMINO-BENZENESULFONYLAMINO)-PIPERIDINE-1- carboxylic acid ethyl ester, which has the structural formula o Oq, CH3 H3CH2CON O I NH aN, S NH H, was prepared as follows. To a suspension of ethyl 4- AMINO-1-PIPERIDINECARBOXYLATE (5.00 g, 29.0 MMOL) and sodium acetate (5.95 G, 72.6 MMOL) in ethanol (58 mL) at 0 C was added N-acetylsulfanilyl chloride (6.10 g, 26.1 MMOL). The mixture stirred at ambient temperature for one hour, then was diluted with water (400 mL) and filtered. The isolated white solid washed with water, dried under vacuum, and used without any further purification. 1H NMR (CD30D) : 8 7.82 (2H, d, J = 8.8 Hz), 7.76 (2H, d, J = 8.8 Hz), 4.08 (2H, q, J = 7.1 Hz), 3.91 (1H, dt, J = 3. 0,13. 8 HZ), 3.34-3. 30 (2H, m), 3.23 (1H, TT, J=4. 1,10. 3 HZ), 2.88 (2H, t, J = 10.3 Hz), 1.73-1. 63 (2H, m), 1.40-1. 27 (2H, m), 1.23 (3H, t, J = 7.1 Hz). 4-AMINO-N-PIPERIDIN-4-YL-BENZENESULFONAMIDE, which has the structural formula NH2 HO OS I Htt o H, was prepared as follows. 4- (4-Acetylamino- benzenesulfonylamino)-piperidine-1-carboxylic acid ethyl ester was dissolved in conc. HCI (60 mL), heated at reflux for 7 hours, allowed to cool, concentrated in vacuo, and dissolved in water (20 mL). Basified to PH=11 with 4N NAOH and extracted with 30% IPROH/CHCI3. The organic layer was dried over NA2SO4 and concentrated to give 2.56 G of white solid (38% for two steps, from N-acetylsulfanilyl chloride), which was used without any further purification. 1H NMR (DMSO-D6) : 8 7.42 (2H, d, J = 8.7 Hz), 7.16 (1 H, d, J = 7.2 Hz), 6.58 (2H, d, J = 8.7 Hz), 5.87 (2H, s), 3.32 (1H, bs), 2.78 (2H, dt, J = 3.9, 12.6 Hz), 2.28 (2H, td, J = 2.1, 11.6 Hz), 1.45 (2H, dd, J = 2. 8,12. 6 Hz), 1.15 (2H, qd, J = 3. 9,11. 6 Hz). FABMS. (MH+) : 256. 4- (4-AMINO-BENZENESULFONYLAMINO)-PIPERIDINE-1-CARBOXYLIC acid t-butyl ester, which II NH2 ZON N? has the structural formula H, was prepared as follows. Triethylamine (0.66 mL, 4.7 MMOL) and di t-butyl dicarbonate (1.13 g, 5.17 MMOL) were sequentially added to a solution OF 4-AMINO-N-PIPERIDIN-4-YL-BENZENESULFONAMIDE (1. 20 g, 4.70 MMOL) in THF (16 mL) and CH2CI2 (16 mL) at 0 C. The mixture was allowed to warm to ambient temperature and stir overnight. The resultant mixture was extracted with CH2CI2. The organic layer was separated, washed with 0.5 N HCI, dried over NA2SO4, and concentrated to give 1.37 G (82% yield) of white solid, which was used without any further purification. 1H NMR (DMSO-d6) : 8 7.43 (2H, d, J = 8.7 Hz), 7.25 (1 H, d, J = 7.3 Hz), 6.59 (2H, d, J = 8.7 Hz), 3.69 (2H, bd, J = 13.4 Hz), 3.02 (1H, m), 2.76 (2H, bs), 1.52 (2H, dd, J = 3.6, 13.4 Hz), 1.36 (9H, s), 1.16 (2H, qd, J = 4.2, 10.3 Hz). 4- (4-ISOTHIOCYANATO-BENZENESULFONYLAMINO)-PIPERIDINE-1-CARBOXYLIC acid t-butyl ester, 0,I zon , was prepared as L JL ' which has the structural formula follows. Thiophosgene (121 mL) was added in one portion to a solution OF 4- (4-AMINO- BENZENESULFONYLAMINO)-PIPERIDINE-1-CARBOXYLIC acid t-butyl ester (562 mg, 1.58 MMOL) in 1 N HCI (4 mL) and THF (4 mL). The mixture stirred for 20 minutes, then partitioned between ether and water. The organic layer was separated, washed with water and brine, dried over NA2SO4, and evaporated to give 578 mg (92% yield) of yellow powder. 1H NMR (DMSO-d6) : 8 7.91 (1 H, d, J = 7.4 Hz), 7.86 (2H, d, J = 8.7 Hz), 7.62 (2H, d, J = 8.7 Hz), 3.71 (2H, bd, J = 13.2 Hz), 3.17 (1H, m), 2.76 (2H, bs), 1. 56-1. 48 (2H, m), 1.36 (9H, s), 1.18 (2H, qd, J = 4. 1,11. 2 Hz). 4-(4-{4-Amino-5-(2,6-difluoro-benzoyl)-thiazol-2-ylamino}-benzenesulfonylamino)- PIPERIDINE-1-CARBOXYLIC acid t-butyl ester, which has the structural formula H2N O F H o X S-O-NH Nu H O , was prepared in a manner similar to that for 4- [4- AMINO-5- (2-HYDROXY-2-METHYL-PROPIONYL)-THIAZOL-2-YLAMINO]-BENZENESULFONAMIDE (EXAMPLE M (1)). 4- (4-ISOTHIOCYANATO-BENZENESULFONYLAMINO)-PIPERIDINE-1-CARBOXYLIC acid t-butyl ester (1.43 G, 3.60 MMOL) LED to 1.52 G (80% yield) of a yellow solid, which was used without further purification. 1H NMR (DMSO-d6) : 8 11.21 (1H, s), 8.25 (2H, bs), 7.80 (4H, s), 7.72 (1H, d, J = 7.3 Hz), 7.58 (1H, m), 7.25 (2H, dd, J = 7. 8,8. 1 Hz), 3.71 (2H, bd, J = 13. 2 HZ), 3.18 (1H, m), 2.80 (2H, bs), 1.55 (2H, dd, J = 3.3, 13.2 Hz), 1.38 (9H, s), 1.21 (2H, qd, J = 3.9, 10. 5 Hz). The title compound was prepared in a manner similar to that for Example D (1). 4- (4- {4-Amino-5-(2,6-difluoro-benzoyl)-thiazol-2-ylamino}-benzenesulfonylamino)-piperidine-1- carboxylic acid t-butyl ester (1. 50 g, 2,8 MMOL) furnished 0.80 G (59% yield) of a yellow solid. 1H NMR (DMSO-d6) : S 8.13 (2H, bs), 7.73 (2H, d, J = 8.9 Hz), 7.66 (2H, d, J = 8.9 Hz), 7.61 (1H, b), 7.52 (1H, m), 7.19 (2H, dd, J = 7. 7,8. 2 HZ), 3.00 (1H, m), 2.84 (2H, bd, J = 12.5 Hz), 2.40 (2H, t, J = 11.0 Hz), 1.51 (2H, d, J = 12. 5 Hz), 1.23 (2H, qd, J = 3.9, 11.0 Hz). HRFABMS: calcd. for C21H22N5O3F2S2 (M+H+) : 494.1132. Found: 494. 1114. Anal. calcd. for C2IHZINS03F2S2 0. 6 H20 0. 3 EtOH : C, 50.07 ; H, 4.67 ; N, 13.52 ; S, 12.38. Found: C, 50.19 ; H, 4.71 ; N, 13.44 ; S, 12.47.
References:
WO2004/72070,2004,A1 Location in patent:Page 93-95