| Identification | More | [Name]
tert-Butyl cyanoacetate | [CAS]
1116-98-9 | [Synonyms]
CYANOACETIC ACID TERT-BUTYL ESTER
T-BUTYL CYANOACETATE
TERT-BUTYL CYANOACETATE
aceticacid,cyano-,1,1-dimethylethylester
Cyanoaceticacidbutylester
3-Oxo-3-tert-butoxypropanenitrile
Cyanoacetic acid 1,1-dimethylethyl ester | [EINECS(EC#)]
214-243-7 | [Molecular Formula]
C7H11NO2 | [MDL Number]
MFCD00001938 | [Molecular Weight]
141.17 | [MOL File]
1116-98-9.mol |
| Safety Data | Back Directory | [Hazard Codes ]
Xn | [Risk Statements ]
R22:Harmful if swallowed. | [Safety Statements ]
S24/25:Avoid contact with skin and eyes . | [WGK Germany ]
3
| [F ]
10-21 | [TSCA ]
Yes | [HS Code ]
29269090 |
| Hazard Information | Back Directory | [Chemical Properties]
Yellow liquid | [Uses]
tert-Butyl cyanoacetate is used in the synthesis of vinylogous urea. It is also used as a new additive for the sugar nucleoside base coupling step en route to DAPD with improved β-selectivity. | [General Description]
tert-Butyl cyanoacetate undergoes functionalization and decarboxylation to form 3-amino-4-alkyl isoquinolines. | [Synthesis]
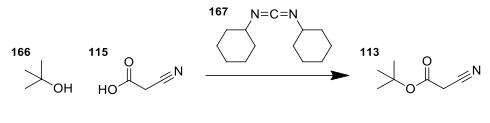
tert-butyl cyanoacetate synthesis: 2-Cyanoacetic acid (1.701 g, 20 mmol) was suspended in a cetonitrile (20 ml) and tert-butanol (2.391 ml, 25.00 mmol). To this, a solution of N,N′-Dicyclohexylcarbodiimide (4.54 g, 22.00 mmol) in DCM (22 ml) was added under stirring at RT. The reaction mixture was stirred for 30 minutes, then filtered through Celite, and the solvents were evaporated. Proton NMR analysis of the residue showed complete conversion to the product. The product, tert-butyl cyanoacetate was not purified, but was taken straight through to the next reaction.
1H NMR (400 MHz, CDCl 3 ) δ 3.37 (s, 2H), 1.50 (s, 9H). | [Purification Methods]
The IR spectrum of a film should have bands at 1742 (ester CO) and 2273 (CN), but no band at ca 3500 broad (OH) cm-1 . If it does not have the last-named band, then fractionally distil; otherwise dissolve in Et2O, wash with saturated aqueous NaHCO3, dry over K2CO3, evaporate Et2O, and distil the residue under a vacuum (see tert-butyl ethyl malonate for precautions to avoid decomposition during distillation). [Beech & Piggott J Chem Soc 423 1955, Dahn & Hauth Helv Chim Acta 42 1214 1959, Beilstein 2 I 255.] |
|
|