| Identification | More | [Name]
MOLYBDENUM(IV) SULFIDE | [CAS]
1317-33-5 | [Synonyms]
MOLYBDENITE
MOLYBDENUM(+4)SULFIDE
Molybdenum(Ⅳ)sulfide
MOLYBDENUM DISULFIDE
MOLYBDENUM DISULPHIDE
MOLYBDENUM(IV) SULFIDE
MOLYBDENUM SULFIDE
dag325
disulfuredemolybdene
molybdenumsulfide(mos2)
molykote
mopolm
mopols
mos2
naturalmolybdenite
MOLYBDENIUM DISULFIDE
MOLYBDENUM DISULFID
MOLYBDENUM(IV) SULFIDE POWDER EXTRA PU&
MOLYBDENUM(IV) SULFIDE, POWDER, <2 MICRO N, 99%
MOLYBDENUM(IV) SULFIDE, POWDER | [EINECS(EC#)]
215-263-9 | [Molecular Formula]
MoS2 | [MDL Number]
MFCD00003470 | [Molecular Weight]
160.07 | [MOL File]
1317-33-5.mol |
| Chemical Properties | Back Directory | [Definition]
Natural molybdenum sul-fide found in igneous rocks and metallic veins. | [Appearance]
dark grey or black powder | [Melting point ]
2375 °C | [density ]
5.06 g/mL at 25 °C(lit.)
| [solubility ]
insoluble in H2O; soluble in concentrated acid solutions | [form ]
powder
| [color ]
Gray to dark gray or black | [Specific Gravity]
4.8 | [Stability:]
Stable. Incompatible with oxidizing agents, acids. | [Water Solubility ]
Soluble in hot sulfuric acid, and aquaregia. Insoluble in water, concentrated sulfuric acid and dilute acid. | [Merck ]
14,6236 | [Boiling point ]
100°C (water) | [Exposure limits]
ACGIH: TWA 10 mg/m3; TWA 3 mg/m3
NIOSH: IDLH 5000 mg/m3 | [InChIKey]
CWQXQMHSOZUFJS-UHFFFAOYSA-N | [Uses]
Molybdenum disulfide (MoS2) is one of the most widely used lubricants in space systems.it is a common additive that improves the antiseize properties of wheel bearing grease.Molybdenum disulfide (MoS2) has been used for many years as a solid lubricant because of its interesting friction-reducing properties related to its crystalline structure. MoS2 is a lamellar compound made of a stacking of S-Mo-S layers . In each of them, the molybdenum atom is surrounded by six sulfur atoms located at the top of a trigonal prism. The distance between a molybdenum atom and a sulfur atom is equal to 0.241 nm, whereas the distance between two sulfur atoms from two adjacent layers is equal to 0.349 nm. This characteristic was often used to explain easy cleavage between the layers and therefore the lubricating properties of MoS2. | [CAS DataBase Reference]
1317-33-5(CAS DataBase Reference) | [EPA Substance Registry System]
Molybdenum sulfide (MoS2) (1317-33-5) | [Bandgap]
1.23 eV | [Chemical Synthesis]
High quality molybdenum disulfide (MoS2) few-layer?films were grown directly on the substrates (SiO2/Si and Sapphire) by chemical vapour deposition (CVD) method. The films were later transferred to the desired substrates?using wet chemical transfer process. | [Electronic properties]
2D Semiconductor | [Odor]
Transparent | [Preparation]
Synthetic?- Chemical Vapour Transport (CVT) |
| Questions And Answer | Back Directory | [Description]
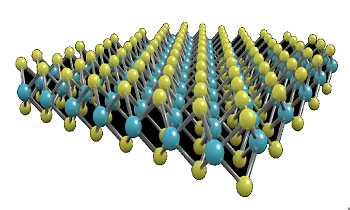
Molybdenum disulfide (MoS2) is the most famous of the single layer transition metal dichalcogenide (TMD) family. MoS2 has been used in bulk for many years as a solid state lubricant, this is due to its low coefficient of friction in addition to its high chemical and thermal stability. All forms of MoS2 have a layered structure, in which a plane of molybdenum atoms is sandwiched by planes of sulfide ions. These three strata form a monolayer of MoS2. Bulk MoS2 consists of stacked monolayers, which are held together by weak van der Waals interactions.

| [structure and hydrogen bonding]
Molybdenum disulfide belongs to a class of materials called 'transition metal dichalcogenides' (TMDCs). Materials in this class have the chemical formula MX2, where M is a transition metal atom (groups 4-12 in the periodic table) and X is a chalcogen (group 16).
The chemical formula of molybdenum disulfide is MoS2. The crystal structure of molybdenum disulfide (MoS2) takes the form of a hexagonal plane of S atoms on either side of a hexagonal plane of Mo atoms. These triple planes stack on top of each other, with strong covalent bonds between the Mo and S atoms, but weak van der Waals forcing holding layers together. This allows them to be mechanically separated to form 2-dimensional sheets of MoS2.
| [Application]
MoS2 with particle sizes in the range of 1-100 μm is a common dry lubricant. Few alternatives exist that can confer the high lubricity and stability up to 350 °C in oxidizing environments. Sliding friction tests of MoS2 using a pin on disc tester at low loads (0.1-2 N) give friction coefficient values of <0.1.
Molybdenum disulfide is often a component of blends and composites where low friction is sought. A variety of oils and greases are used, because they retain their lubricity even in cases of almost complete oil loss, thus finding a use in critical applications such as aircraft engines. When added to plastics, MoS2 forms a composite with improved strength as well as reduced friction. Polymers that have been flld with MoS2 include nylon (with the trade name Nylatron), Teflon, and Vespel. Self-lubricating composite coatings for high-temperature applications have been developed consisting of molybdenum disulfide and titanium nitride by chemical vapor deposition.
MoS2 is often used in two-stroke engines; e.g. motorcycle engines. It is also used in CV and universal joints. MoS2-coatings allow bullets easier passage through the rifle barrel causing less barrel fouling allowing the barrel to retain ballistic accuracy much longer. This resistance to barrel fouling comes at a cost of lower muzzle velocity with the same load due to a decreased chamber pressure. MoS2 is applied to bearings in ultra- high vacuum applications up to 10-9 torr (at -226 to 399 °C). The lubricant is applied by burnishing and the excess is wiped from the bearing surface.
MoS2 is also used in ski wax to prevent static buildup in dry snow conditions and to add glide when sliding in dirty snow. It is often used in two-stroke engines; e.g., motorcycle engines. MoS2 is also used in CV and universal joints. During the Vietnam War, the molybdenum disulfide product "Dri-Slide" was used to lubricate weapons, although it was supplied from private sources, not the military. MoS2 -coatings allow bullets easier passage through the rifle barrel with less deformation and better ballistic accuracy.
| [Synthesis]
The preparation of MoS2 was carried out through modification of the method described in literature. All the chemicals were purchased and used as received. To start, 30 mL of 0.008 M ammonium molybdate ((NH4)6Mo7O24·4H2O, Merck India, 98%) solution was taken, and sodium dodecyl sulfate (SDS) of 10 times of cmc (critical micelle concentration) was added to it under constant stirring to obtain a clear solution. Then, 9.60 mL of 0.23 M sodium dithionite (Na2S2O4, BDH, England, 98% pure) solution and 45 mL of 0.20 M thioacetamide (CH3CSNH2, Spectrochem India, 99%) solution were added into the former solution and were thoroughly mixed together by stirring. The solution mixture was heated (~90°C) over a water bath to obtain a clear reddish yellow color solution. Acidification of this solution with concentrated HCl (pH < 1) led to a dark brown colored precipitate. The precipitate was isolated using a centrifuge and was washed with water for several times. Drying of the precipitate gave rise to brownish black powders, which were calcined at 400°C for 2 h under argon atmosphere to obtain the black powders of MoS2. |
| Hazard Information | Back Directory | [Hazard]
Toxic material. | [Chemical Properties]
dark grey or black powder,Molybdenum disulfide, MoS2, the most common natural form of molybdenum, is extracted from the ore and then purified for direct use in lubrication. Since molybdenum disulfide is of geothermal origin, it has the durability to withstand heat and pressure. This is particularly so if small amounts of sulfur are available to react with iron and provide a sulfide layer which is compatible with MoS2 in maintaining the lubricating film.
| [General Description]
Molybdenum disulfide is a two dimensional layered material. Monolayers of transition metal dichalcogenides (TMDs)exhibit photoconductivity. The layers of the TMD can be mechanically or chemicaly exfoliated to form nanosheets. |
|
|