| Identification | Back Directory | [Name]
FrovatriptanSuccinate | [CAS]
158930-09-7 | [Synonyms]
Vml 251
Sb-209509ax
Fovatriptan succinate
FrovatriptanSuccinate
(R)-3-(Methylamino)-2,3,4,9-tetrahydro-1H-carbazole-6-carboxamide succinate
(3R)-2,3,4,9-Tetrahydro-3-(methylamino)-1H-carbazole-6-carboxamide butanedioate
butanedioic acid,(6R)-6-(methylamino)-6,7,8,9-tetrahydro-5H-carbazole-3-carboxamide
1H-Carbazole-6-carboxamide, 2,3,4,9-tetrahydro-3-(methylamino)-, (R)-, butanedioate (1:1)
1H-Carbazole-6-carboxamide, 2,3,4,9-tetrahydro-3-(methylamino)-, (3R)-, butanedioate (1:1)
Butanedioic acid, compd. with (3R)-2,3,4,9-tetrahydro-3-(methylamino)-1H-carbazole-6-carboxamide (1:1) | [Molecular Formula]
C18H23N3O5 | [MOL File]
158930-09-7.mol | [Molecular Weight]
361.4 |
| Chemical Properties | Back Directory | [storage temp. ]
Store at -20°C | [solubility ]
DMSO: 72 mg/mL (199.23 mM);Ethanol: Insoluble | [Water Solubility ]
Water: 72 mg/mL (199.23 mM) |
| Hazard Information | Back Directory | [Description]
Frovatriptan succinate was launched as an oral treatment for acute migraine attacks
with
or without aura in adults. It is the eighth member of the “triptan” class. Frovatriptan is a
conformationally-restricted analog of the 5-HT1-receptor agonist 5-carboxytryptamine
which can be prepared in six steps. The key intermediate (R)-6-cyano-3-N-methylamino-
1,2,3,4-tetrahydrocarbazole is obtained by Fischer reaction of 4-cyanophenylhydrazine
with the appropriate ketone followed by resolution using L-pyroglutamic acid. This drug
acts as a dual 5-HT1D/1B receptor partial agonist and has high and selective affinity for 5HT1B and 5-HT1D receptors in cranial vessels. It has no significant activity at 5-HT2, 5-HT3,
5-HT4, α-adrenergic, histaminergic or GABAA receptors. Frovatriptan is also a moderately
potent full agonist at 5-HT7 receptors, which have a dilatory action and are expressed in
the human coronary artery. In vitro studies appear to indicate frovatriptan’s functional
selectivity for cerebral circulation as shown by the concentrations needed to induce
threshold contractile activity and maximum response in basilar arteries as compared with
coronary arteries. Frovatriptan is mainly metabolized by CYPlA2 and most of its
metabolites are excreted renally. Co-administration of frovatriptan with the monoamine
oxidase-A inhibitor moclobemide or with the potent CYPlA2 inhibitor fluvoxamine did not
affect its pharmacokinetics parameters. Although frovatriptan has a poor bioavailability (24-
30%), it has a very long half-life compared to other triptans (25 h) and has an onset of
action and efficacy similar to those of naratriptan. The most striking features of this drug
are the low headache recurrence rate, which is one of the lowest among the triptans and
which may be attributed to its long half-life, and excellent tolerance profile. No significant
effect on the cardiovascular system was seen after administration of a single oral dose of
14 fold the therapeutic dose of frovatriptan. | [Originator]
GlaxoSmithKlineNernaIis (UK) | [Manufacturing Process]
4-Carboxamidophenylhydrazine hydrochloride (2.87 g) and 4-
phthalimidocyclohexanone (3.00 g) were mixed in acetic acid and the mixture
was heated under reflux for 2 h. After cooling, the mixture was neutralized
using aq. potassium carbonate solution, and the yellow solid thus obtained
was filtered, washed with water, and dried. Purification by column
chromatography (SiO2; CHCl3/CH3OH) gave 6-carboxamido-3-phthalimido-
1,2,3,4-tetrahydrocarbazole (2.8 g).
The 6-carboxamido-3-phthalimido-1,2,3,4-tetrahydrocarbazole (1.0 g) was
suspended in ethanol (10 ml) and hydrazine hydrate (5 ml) was added. A
clear solution was obtained, and the mixture was left to stir overnight, to yield
a precipitate. The whole mixture was evaporated to dryness, washed with aq.
K2CO3 solution, and water, to leave the (+/-)-3-amino-6-carboxamido-1,2,3,4-
tetrahydrocarbazole (0.44 g), melting point 146°-148°C.
Separation of diastereoisomers of a chiral derivative of a 3-amino-6-
carboxamido-1,2,3,4-tetrahydrocarbazole e.g. by crystallisation, or by
chromatography.
Benzaldehyde (10.6 g) was added to a suspension of (+)-3-amino-6-
carboxamido-1,2,3,4-tetrahydrocarbazole (12.35 g) in methanol (100 ml). The
mixture was stirred for 1 h, sodium cyanoborohydride (9.3 g) added over 1 h
and the clear solution stirred for 24 h. The solution was cooled (ice bath) and
formaldehyde (37% aqueous methanolic, 9:1 solution, 5.5 ml) added. After
30 min stirring at room temperature water (100 ml) was added, stirring
continued for 30 min followed by extraction with dichloromethane (3 times
150 ml). The combined organic extracts were washed with water (2 times 200
ml), dried (Na2SO4), filtered and solvent removed at reduced pressure. The
residue was column chromatographed (silica gel, dichloromethane-10%
ethanol/dichloromethane) to give 3-N-benzyl-6-carboxamido-3-N�methylamino-1,2,3,4-tetrahydrocarbazole (9.4 g) as a foam. The succinate salt (1:1) was recrystallised from methanol, melting point 175°-182°C.
To a solution of 3-N-benzyl-6-carboxamido-3-N-methylamino-1,2,3,4-
tetrahydrocarbazole (1.0 g) in ethanol (100 ml) containing succinic acid (0.39
g), Pearlmans catalyst (1.0 g) was added and the mixture shaken under an
atmosphere of hydrogen at 45 psi and 50°C for 2 h. The mixture was filtered
(celite pad) and the pad washed thoroughly with ethanol. The combined
flitrate and washings were evaporated to dryness, coevaporated with ethanol
(3 times 100 ml) and recrystallised from methanol to give the (+)-6-
carboxamido-3-N-methylamino-1,2,3,4-tetrahydrocarbazole succinate (1:1)
salt, melting point 148°-155°C. | [Brand name]
Frova | [Therapeutic Function]
Migraine therapy | [Synthesis]
The synthesis of frovatriptan (11)
appeared in a patent in multi-kilo scale.
Cyclohexanedione monoketal (121) was converted to amine
122 by reductive amination. The Fischer indolization of
amine 122 with hydrazine 123 furnished indole nitrile 124
in 72% yield. The desired R isomer of the indole nitrile was
obtained via a chiral salt formation/recrystallization process
using chiral lactam 125 and isolated as a L-pyroglutamic
acid salt 126. Hydrolysis of the nitrile functional group in
126 provided carboxamido indole 127, which was converted
to succinate 11 in situ.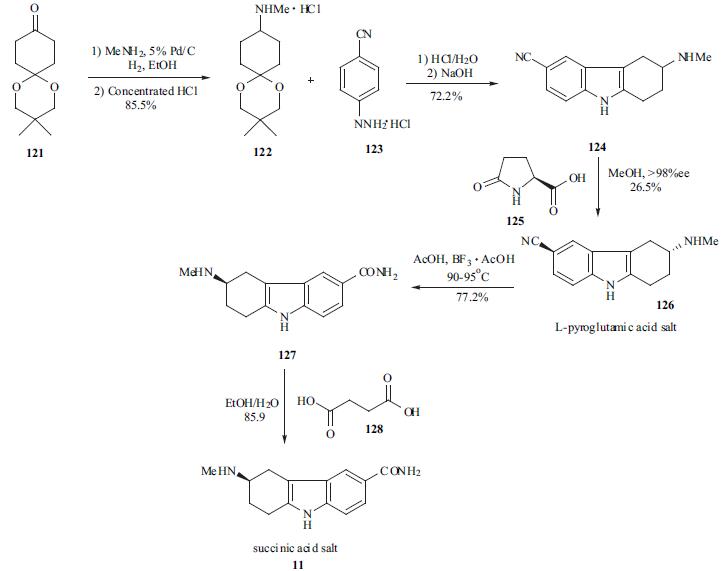
| [storage]
Store at -20°C |
|
| Company Name: |
Nosch Labs Pvt Ltd
|
| Tel: |
+91-4048557474 +91-9948222287 |
| Website: |
www.noschlabs.net |
|