| Identification | More | [Name]
2-Methyl-3-nitropyridine | [CAS]
18699-87-1 | [Synonyms]
2-METHYL-3-NITROPYRIDINE
3-NITRO-2-METHYLPYRIDINE
3-NITRO-2-PICOLINE
Pyridine, 2-methyl-3-nitro-(9CI)
3-Nitro-2-methylpyridine
3-Nitro-2-picoline
2-METHYL-3-NITROPYRIDINE 95%
3-NITROPICOLINE | [EINECS(EC#)]
805-108-9 | [Molecular Formula]
C6H6N2O2 | [MDL Number]
MFCD04114137 | [Molecular Weight]
138.12 | [MOL File]
18699-87-1.mol |
| Chemical Properties | Back Directory | [Melting point ]
32-33° | [Boiling point ]
86°C/5mmHg(lit.) | [density ]
1.246±0.06 g/cm3(Predicted) | [storage temp. ]
under inert gas (nitrogen or Argon) at 2-8°C | [solubility ]
soluble in Methanol | [form ]
powder to lump | [pka]
1.92±0.10(Predicted) | [color ]
Light yellow to Brown | [InChI]
InChI=1S/C6H6N2O2/c1-5-6(8(9)10)3-2-4-7-5/h2-4H,1H3 | [InChIKey]
CCFGTKQIRWHYTB-UHFFFAOYSA-N | [SMILES]
C1(C)=NC=CC=C1[N+]([O-])=O | [CAS DataBase Reference]
18699-87-1(CAS DataBase Reference) |
| Hazard Information | Back Directory | [Uses]
2-Methyl-3-nitropyridine is used as a starting reagent to synthesize 3-substituted-4 or 6-azaindoles, and is also used as a reagent to prepare 3-azaindolyl-4-arylmalemides (compounds that exhibit antiproliferative activity). | [Reactions]
2-Methyl-3-nitropyridine could be used to synthesize dichloro-(3-nitro-2-pyridyl)methylphosphonic dichloride. The chlorination of 2-methyl-3-nitropyridine using a mixture of PCl5 in POCl3[1].
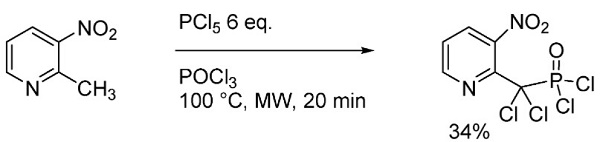 | [Synthesis Reference(s)]
Tetrahedron, 54, p. 6311, 1998 DOI: 10.1016/S0040-4020(98)00328-7
Journal of Heterocyclic Chemistry, 29, p. 359, 1992 DOI: 10.1002/jhet.5570290213
Synthetic Communications, 20, p. 2965, 1990 DOI: 10.1080/00397919008051513 | [Synthesis]
Suzuki Reaction. A mixture of 2-chloro-3-nitro-pyridine 1 (793 mg, 5 mmol), methylboronic acid (329 mg, 5.5 mmol), Pd(PPh3) 4 (578 mg, 0.5 mmol) and K2CO 3 (2.073 g, 15 retool) in dioxane (25 mL) was refluxed for 2 days, then cooled to room temperature and filtered. The solvent was removed and the residue was isolated by chromatography (hexanes-EtOAc = 1:1) to provide 623 mg (90%) of 2-methyl-3-nitropyridine[2].
| [References]
[1] Amrane D, et al. "Dichloro{4-(4-chlorophenoxy)phthalazin-1-yl} methylphosphonic dichloride." Molbank (2022).
[2] Niu, Chuansheng , et al. "Synthesis of 3-aminopyridine-2-carboxaldehyde thiosemicarbazone (3-AP)." 54.23(1998):6311-6318. |
|
|