| Identification | Back Directory | [Name]
1-[AMINO(PHENYL)METHYL]-2-NAPHTHOL HYDROCHLORIDE | [CAS]
481-82-3 | [Synonyms]
1-(aminophenylmethyl)-2-Naphthalenol
2-Naphthalenol, 1-(aminophenylmethyl)-
1-[azanyl(phenyl)methyl]naphthalen-2-ol
2-[(E)-3-phenylprop-2-enylidene]propanedioic acid | [Molecular Formula]
C17H15NO | [MOL File]
481-82-3.mol | [Molecular Weight]
249.31 |
| Chemical Properties | Back Directory | [Melting point ]
173-175 °C(Solv: ethanol (64-17-5)) | [Boiling point ]
451.1±30.0 °C(Predicted) | [density ]
1.211±0.06 g/cm3(Predicted) | [pka]
9.07±0.50(Predicted) |
| Hazard Information | Back Directory | [Uses]
2-Naphthalenol, 1-(aminophenylmethyl)- can be used as organic synthesis intermediate and pharmaceutical intermediate, mainly used in laboratory research and development process and chemical production process. | [Synthesis]
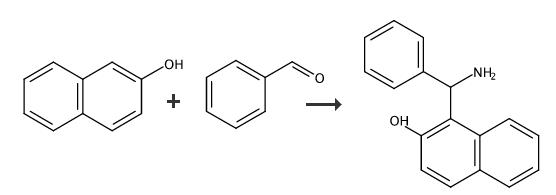
Step 1
General/Typical Procedure: General experimental procedures Method A: for compounds 3 (a-i). A mixture of aromatic/heteroaromatic aldehydes (2 mmol), 2-naphthol (1 mmol), and ammonium acetate (1.5 mmol) were taken in a beaker. The reaction mixture was hom ogenized with the help of glass rod and irradiated in microwave oven (360 W) by interval of 10 second. The progress of the reaction was monitored by TLC . After completion, the reaction mixture was cooled to room temperature and was poured over crushed ice. The obtained solid was filtered, dried, and recrystallized from ethanol. 1,3 oxazine derivative 3a, Yield: 98%, Time: 5 minutes. M.P: 147-149°C.
Step 2
General/Typical Procedure: General experim ental procedures for compounds 4 (a,d-f). Compounds 3(a, d-f) (1 mmol) were taken in 2 ml dil HCl in a beaker and followed by irradiation in a microwave (360 W) for about 5 min. KOH (0.2 mmol) was added and was then irradiated for the appropriate time until the completion of the reaction. The progress of the reaction was monitored by TLC. After completion, the reaction mixture was cooled at room temperature and was poured on crushed ice. The obtained solid was filtered, dried, and recrystallized from ethanol. Aminobenzyl naphthol (Betti base) 4a, Yield: 95%, Time: 15 minutes. M.P: 173-175°C. |
|
| Company Name: |
Enamine
|
| Tel: |
(380) 44 537 32 18 |
| Website: |
www.enamine.net |
|