2-Carboxyethylacrylat Chemische Eigenschaften,Einsatz,Produktion Methoden
R-Sätze Betriebsanweisung:
R34:Verursacht Verätzungen.
R36/37/38:Reizt die Augen, die Atmungsorgane und die Haut.
S-Sätze Betriebsanweisung:
S23:Gas/Rauch/Dampf/Aerosol nicht einatmen(geeignete Bezeichnung(en) vom Hersteller anzugeben).
S26:Bei Berührung mit den Augen sofort gründlich mit Wasser abspülen und Arzt konsultieren.
S36/37/39:Bei der Arbeit geeignete Schutzkleidung,Schutzhandschuhe und Schutzbrille/Gesichtsschutz tragen.
S45:Bei Unfall oder Unwohlsein sofort Arzt zuziehen (wenn möglich, dieses Etikett vorzeigen).
S36:DE: Bei der Arbeit geeignete Schutzkleidung tragen.
Chemische Eigenschaften
Colorless to light yellow viscid liquid
Verwenden
2-Carboxyethyl Acrylate is used in the preparation of DNase enzyme derivatives that act as potent preventative material of bacterial adhesion and biofilm formation in biomaterials.
Application
2-Carboxyethyl acrylate can be polymerized in solution or emulsion to produce acrylic, vinyl acrylic, or styrene acrylic polymers, which are distinguished by their low glass transition temperatures (<30°C) as homopolymers. Greater elasticity, as well as improved adhesion.
synthetische
Synthesis of 1-ethoxyethyl acrylate (EEA) and protected 2-carboxyethyl acrylate (proCEA)
EEA and proCEA were synthesized following a previously published procedure and distilled prior to use. For the synthesis of proCEA (Figure 1), phosphoric acid (109 mg, 1.11 mmol) was weighed into a dry round bottom flask in a glovebox and then taken outside the glovebox, taking care that the phosphoric acid stayed dry. 2-Carboxyethyl acrylate (80 g, 555 mmol) and ethyl vinyl ether (48 g, 666 mmol) were added and the reaction was stirred for two days at room temperature. Hydrotalcite (Mg6Al2(OH)16CO3·4H2O, ~1 g) was added, stirred for one hour and filtered off. Excess ethyl vinyl ether was removed under reduced pressure and the product was distilled under reduced pressure (80 °C, 1.3 mbar).
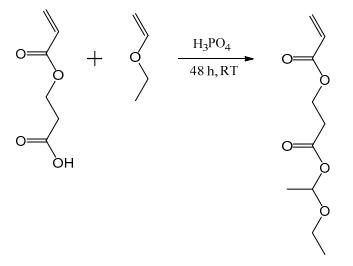
Figure 1 Synthesis of proCEA
Hazard
A severe skin irritant.
Einzelnachweise
[1] Emilia Pietrzak, Mikolaj Szafran, Paulina Wiecinska. “2-carboxyethyl acrylate as a new monomer preventing negative effect of oxygen inhibition in gelcasting of alumina.” Ceramics International 42 12 (2016): Pages 13682-13688.
[2] Naveed Ullah . “Coupling of carboxymethyl starch with 2-carboxyethyl acrylate: A new sorbent for the wastewater remediation of methylene blue.” Environmental Research 219 (2023): Article 115091.
[3] Amit K. Tripathi, Donald C. Sundberg*, Jenna Vossoughi. “Partitioning of 2-Carboxyethyl Acrylate between Water and Vinyl Monomer Phases Applied to Emulsion Polymerization: Comparisons with Hydroxy Acrylate and Other Vinyl Acid Functional Monomers.” Industrial Engineering Chemistry Research 54 9 (2015): 2447–2452.
2-Carboxyethylacrylat Upstream-Materialien And Downstream Produkte
Upstream-Materialien
Downstream Produkte

