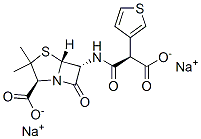
Ticarcillin sodium
- CAS No.
- 74682-62-5
- Chemical Name:
- Ticarcillin sodium
- Synonyms
- TICAR;TRIARCILLINSODIUM;Ticacillin sodium;Ticarcillin sodium;TICARCILLIN DISODIUM;Ticarcillin MonosodiuM Monohydrate;Ticarcillin Monosodium Monohydrate (200 mg);Ticarcillin Monosodium Monohydrate (1667304);TICARCILLIN SODIUM EPT(CRM STANDARD) USP/EP/BP;(2S,5S,6R)-6-[[(2R)-CARBOXY-3-THIENYLACETYL]AMINO]-3,3-DIMETHYL-7-OXO-4-THIA-1-AZABICYCLO[3,2,0]HEPT
- CBNumber:
- CB7147337
- Molecular Formula:
- C15H14N2Na2O6S2
- Molecular Weight:
- 428.39
- MDL Number:
- MFCD07787410
- MOL File:
- 74682-62-5.mol
| storage temp. | -20°C |
|---|---|
| CAS DataBase Reference | 74682-62-5(CAS DataBase Reference) |
| FDA UNII | SM6JM116PM |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS08 |
|---|---|
| Signal word | Danger |
| Hazard statements | H334-H317 |
| Precautionary statements | P261-P285-P304+P341-P342+P311-P501-P261-P272-P280-P302+P352-P333+P313-P321-P363-P501 |
| HS Code | 2941106000 |
Ticarcillin sodium price More Price(4)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | Y0000465 | Ticarcillin monosodium European Pharmacopoeia (EP) Reference Standard | 74682-62-5 | y0000465 | $154 | 2024-03-01 | Buy |
| Sigma-Aldrich | 1667304 | Ticarcillin monosodium | 74682-62-5 | 350mg | $440 | 2024-03-01 | Buy |
| Arctom | AS033050 | Ticarcillinsodium | 74682-62-5 | 1g | $118 | 2021-12-16 | Buy |
| Arctom | AS033050 | Ticarcillinsodium | 74682-62-5 | 5g | $412 | 2021-12-16 | Buy |
Ticarcillin sodium Chemical Properties,Uses,Production
Originator
Ticar,Beecham,US,1976
Uses
Ticar (Glaxo-SmithKline).
Definition
ChEBI: Ticarcillin disodium is an organic sodium salt. It contains a ticarcillin(2-).
Manufacturing Process
A mixture of monobenzyl-3-thienylmalonate (1.38 g, 5 mmol) and thionyl
chloride (2.5 ml) was warmed at 50°C to 55°C for 1 hour, then at 60°C to
65°C for 10 minutes. The excess of thionyl chloride was removed in vacuo at
not more than 30°C, the last traces being removed by codistillation with dry
benzene (1 ml) under high vacuum, leaving monobenzyl3-thienylmalonyl
chloride as a yellow oil.
The acid chloride obtained as described above was dissolved in dry acetone
(10 ml) and added in a steady stream to a stirred solution of 6-
aminopenicillanic acid (1.08 g, 5 mmol) in a mixture of N sodium bicarbonate
(15 ml) and acetone (5 ml). After the initial reaction the reaction mixture was
stirred at room temperature for 45 minutes, then washed with ether (3 x 25
ml). Acidification of the aqueous solution with N hydrochloric acid (11 ml) to
pH 2 and extraction with ether (3 x 15 ml) gave an ethereal extract which
was decolorized with a mixture of activated charcoal and magnesium sulfate
for 5 minutes.
The resulting pale yellow ethereal solution was shaken with sufficient N
sodium bicarbonate (4 ml) to give an aqueous extract of pH 7 to 7.5. This
extract was concentrated to syrup at low temperature and pressure, then
isopropanol was added with stirring until the mixture contained about 10%
water.
Crystallization was initiated, and completed at about 0°C overnight, to give
the sodium salt of α-(benzyloxycarbonyl)-3-thienylmethylpenicillin as white
crystals in 50% weight yield. This product was estimated by colorimetric assay
with hydroxylamine to contain 91% of the anhydrous sodium salt.
A solution of the sodium salt of α-(benzyloxycarbonyl)-3-
thienylmethylpenicillin (2.13 g, 4.3 mmol) in water (30 ml) was added to a
suspension of 5% palladium on calcium carbonate (10.65 g) in water (32 ml)
which had been prehydrogenated for 1 hour.
The mixture was then hydrogenated at just above atmospheric pressure for 1
1/2 hours and filtered through a Dicalite bed. The clear filtrate was
evaporated at low temperature and pressure, and the residue dried in vacuo
over phosphorus pentoxide, to give 1.64 g of the salt of α-(3-
thienyl)methylpenicillin as a white solid.
Colorimetric assay with hydroxylamine showed this salt to contain 94% of the anhydrous penicillin. Paper chromatography showed complete reduction of the
benzyl group.
Therapeutic Function
Antibiotic
Clinical Use
Ticarcillin disodium, α-carboxy-3-thienylpenicillin (Ticar),is an isostere of carbenicillin in which the phenyl group is replacedby a thienyl group. This semisynthetic penicillinderivative, like carbenicillin, is unstable in acid and, therefore,must be administered parenterally. It is similar tocarbenicillin in antibacterial spectrum and pharmacokineticproperties. Two advantages for ticarcillin are claimed:(a) slightly better pharmacokinetic properties, includinghigher serum levels and a longer duration of action; and(b) greater in vitro potency against several species of Gramnegativebacilli, most notably P. aeruginosa and Bacteroidesfragilis. These advantages can be crucial in the treatment ofserious infections requiring high-dose therapy.
Ticarcillin sodium Preparation Products And Raw materials
Raw materials
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Henan Fengda Chemical Co., Ltd | +86-371-86557731 +86-13613820652 | info@fdachem.com | China | 18232 | 58 |
| Shaanxi TNJONE Pharmaceutical Co., Ltd | +8618740459177 | sarah@tnjone.com | China | 1107 | 58 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | info@tianfuchem.com | China | 21689 | 55 |
| ATK CHEMICAL COMPANY LIMITED | +undefined-21-51877795 | ivan@atkchemical.com | China | 32686 | 60 |
| career henan chemical co | +86-0371-86658258 | sales@coreychem.com | China | 29914 | 58 |
| Chongqing Chemdad Co., Ltd | +86-023-61398051 +8613650506873 | sales@chemdad.com | China | 39916 | 58 |
| CONIER CHEM AND PHARMA LIMITED | +8618523575427 | sales@conier.com | China | 49391 | 58 |
| SIMAGCHEM CORP | +86-13806087780 | sale@simagchem.com | China | 17367 | 58 |
| Shaanxi Dideu Medichem Co. Ltd | +86-029-89586680 +86-18192503167 | 1026@dideu.com | China | 9126 | 58 |
| Dideu Industries Group Limited | +86-29-89586680 +86-15129568250 | 1026@dideu.com | China | 28608 | 58 |
View Lastest Price from Ticarcillin sodium manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2024-04-24 | Ticarcillin sodium
74682-62-5
|
US $8.00-2.00 / kg | 1kg | 99% | g-kg-tons, free sample is available | Henan Fengda Chemical Co., Ltd | |
 |
2024-04-12 | Ticarcillin sodium
74682-62-5
|
US $0.00 / kg | 1kg | 99% | 10000kg | Shaanxi TNJONE Pharmaceutical Co., Ltd | |
 |
2022-05-17 | Ticarcillin sodium
74682-62-5
|
US $1.10 / g | 1g | 99.9% Min | 100 Tons | Dideu Industries Group Limited |
-

- Ticarcillin sodium
74682-62-5
- US $8.00-2.00 / kg
- 99%
- Henan Fengda Chemical Co., Ltd
-

- Ticarcillin sodium
74682-62-5
- US $0.00 / kg
- 99%
- Shaanxi TNJONE Pharmaceutical Co., Ltd
-

- Ticarcillin sodium
74682-62-5
- US $1.10 / g
- 99.9% Min
- Dideu Industries Group Limited