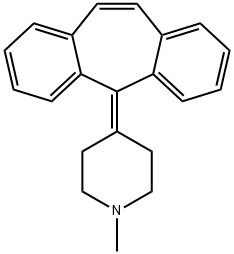
Cyproheptadine
- CAS No.
- 129-03-3
- Chemical Name:
- Cyproheptadine
- Synonyms
- Periactin;MK 141;Dronactin;Periactine;Periactinol;Cypoheptadine;AKOS NCG1-0040;CYPROHEPTADINE;Eiproheptadine;Cycloheptadine
- CBNumber:
- CB7782550
- Molecular Formula:
- C21H21N
- Molecular Weight:
- 287.4
- MDL Number:
- MFCD00242817
- MOL File:
- 129-03-3.mol
| Melting point | 112.3-113.3° |
|---|---|
| Boiling point | 419.7°C (rough estimate) |
| Density | 0.9917 (rough estimate) |
| refractive index | 1.8240 (estimate) |
| storage temp. | Store at -20°C |
| form | Solid |
| pka | pKa 8.87 (Uncertain) |
| color | Crystals from EtOH (aq) |
| Water Solubility | 317.6ug/L(22.5 ºC) |
| CAS DataBase Reference | 129-03-3(CAS DataBase Reference) |
| FDA UNII | 2YHB6175DO |
| NCI Dictionary of Cancer Terms | cyproheptadine |
| NCI Drug Dictionary | Periactin |
| ATC code | R06AX02 |
| NIST Chemistry Reference | Cyproheptadine(129-03-3) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|---|---|
| Signal word | Warning |
| Hazard statements | H315-H319-H335 |
| Precautionary statements | P261-P305+P351+P338 |
| HS Code | 29143900 |
| Toxicity | LD50 orl-rat: 295 mg/kg 27ZQAG -,69,72 |
Cyproheptadine price More Price(12)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Cayman Chemical | 19551 | Cyproheptadine ≥98% | 129-03-3 | 100mg | $39 | 2021-12-16 | Buy |
| Cayman Chemical | 19551 | Cyproheptadine ≥98% | 129-03-3 | 1g | $215 | 2021-12-16 | Buy |
| Cayman Chemical | 19551 | Cyproheptadine ≥98% | 129-03-3 | 250mg | $88 | 2021-12-16 | Buy |
| Cayman Chemical | 19551 | Cyproheptadine ≥98% | 129-03-3 | 500mg | $156 | 2021-12-16 | Buy |
| TRC | H999760 | Cycloheptadine | 129-03-3 | 250mg | $550 | 2021-12-16 | Buy |
Cyproheptadine Chemical Properties,Uses,Production
Description
Cyproheptadine has antianaphylactic activity that is associated with its ability to slow down the release of histamine and other mediators from fat cells.
Originator
Periactin,Merck Sharp and Dohme,US,1961
Uses
It is mainly used for treating bronchial asthma attacks, allergic bronchitis, rhinitis, and allergic skin reactions as well as in adjuvant therapy for anaphylactic reactions. Synonyms of this drug are periactin and vimicon.
Uses
Antihistaminic; antipruritic.
Definition
ChEBI: The product resulting from the formal oxidative coupling of position 5 of 5H-dibenzo[a,d]cycloheptene with position 4 of 1-methylpiperidine resulting in the formation of a double bond between the two fragments. t is a sedating antihistamine with antimuscarinic and calcium-channel blocking actions. It is used (particularly as the hydrochloride sesquihydrate) for the relief of allergic conditions including rhinitis, conjunctivitis due to inhalant allergens and food , urticaria and angioedema, and in pruritic skin disorders. Unlike other antihistamines, it is also a seratonin receptor antagonist, making it useful in conditions such as vascular headache and anorexia.
Manufacturing Process
(A) Preparation of 1-Methyl-4-Piperidyl-Magnesium Chloride: Magnesium
turnings (5.45 g, 0.22 g-atom) were placed in a 500 ml 3-necked flask
provided with a condenser, Hershberg stirrer and dropping funnel and
protected with a drying tube. An atmosphere of dry nitrogen was maintained
in the apparatus throughout the reaction. The magnesium was covered with 20 ml of dry tetrahydrofuran. A crystal of iodine and 1.2 g of ethyl bromide
were added and after the reaction had subsided (formation of ethylmagnesium
bromide) a solution of 29.4 g (0.22 mol) of 4-chloro-1-methyl-piperidine in
dry tetrahydrofuran (total volume, 103 ml) was added dropwise at such a rate
that gentle reflux was maintained.
The solution of 4-chloro-1-methylpiperidine in tetrahydrofuran was dried over
calcium hydride at ice-bath temperature prior to use. When the addition of the
halide was complete the reaction mixture was refluxed with stirring for one
hour. In some subsequent experiments this period of refluxing was omitted
with no deleterious result.
The solution of 4-chloro-1-methylpiperidine in tetrahydrofuran was dried over
calcium hydride at ice-bath temperature prior to use. When the addition of the
halide was complete the reaction mixture was refluxed with stirring for one
hour. In some subsequent experiments this period of refluxing was omitted
with no deleterious result.
The solvent was evaporated from the combined benzene extracts to give 33.4
g of a clear light brown resin. Crystallization from an alcohol-water mixture
gave 19.5 g of 1-methyl-4-(5-hydroxy-5-dibenzo[a,e]cycloheptatrienyl)-
piperidine, MP 156° to 157°C. Two recrystallizations from alcohol-water
mixtures followed by two recrystallizations from benzene-hexane mixtures
gave analytically pure product, MP 166.7° to 167.7°C.
(C) Preparation of 1-Methyl-4-(5-Dibenzo[a,e]Cycloheptatrienylidene)-
Piperidine Hydrochloride: 1-Methyl-4-(5-hydroxy-5-dibenzo[a,e]
cycloheptatrienyl)-piperidine (3.05 g, 0.01 mol) was dissolved in glacial acetic
acid, 15 ml. The solution was saturated with dry hydrogen chloride with
external cooling. A white solid separated. Acetic anhydride (3.07 g, 0.03 mol)
was added and the mixture heated on the steam bath for one hour. The solid
dissolved in the first 5 minutes of the heating period.
The reaction mixture was poured into 25 ml of water and the mixture made
strongly basic with 10N sodium hydroxide solution. The mixture was extracted
3 times with 50 ml portions of benzene, the combined extracts washed with
water and concentrated to a volume of approximately 50 ml. The solution was
saturated with dry hydrogen chloride and the white crystalline product
collected and dried. The yield of product, MP 251.6° to 252.6°C (dec.) was
2.5 g. Recrystallization from a mixture of absolute alcohol and absolute ether
gave a product, MP 252.6° to 253.6°C. A sample was analyzed after drying
for 7 hours at 110°C over phosphorus pentoxide in vacuo.
(D) Preparation of 1-Methyl-4-(5-Dibenzo[a,e]Cycloheptatrienylidene)-
Piperidine: The hydrochloride salt, 4.3 g, was suspended in 100 ml of warm
water and the mixture made strongly alkaline by the addition of 15 ml of 5%
sodium hydroxide. The mixture was extracted with four 50 ml portions of
benzene and the extracts dried over sodium sulfate. Evaporation of the
benzene on the steam-bath at reduced pressure left 3.7 g (97%) of the base,MP 110.3° to 111.3°C. Recrystallization from a mixture of alcohol and water
gave product, MP 112.3° to 113.3°C.
brand name
Periactin (Merck);Anarexal;Antegan;Apeplus;Brantina;Brantine;Carnigol;Carpantin;Ciplactin;Cipractin;Cipro n;Ciprocort;Cyrasarl;Estialim;Histatets;Ifrasarl;Kontrast u;Naidoretico;Nurdelin;Nuttriben;Oractine;Orexigen;Periactol;Perideca;Pranzo;Reparal carnitina;Siglatan;Sigloton;Sipraktin;Siprodin;Vimicon.
Therapeutic Function
Antipruritic, Antihistaminic, Appetite stimulant
World Health Organization (WHO)
Cyproheptadine, an antihistamine with anticholinergic and serotonin-antagonist properties, was introduced in 1961 for the symptomatic relief of allergy and was subsequently used as an appetite stimulant. In 1982 the drug was prohibited in Bangladesh because of its misuse as an appetite stimulant due to inappropriate promotion. Cyproheptadine remains widely available and the current marketing policy of the major manufacturer requires that it should be used as an appetite stimulant only under the supervision of a physician who should be assured that adequate food is available.
Safety Profile
Poison by ingestion, intraperitoneal, subcutaneous and intravenous routes. Experimental teratogenic and reproductive effects. When heated to decomposition it emits toxic fumes of NOx.
Synthesis
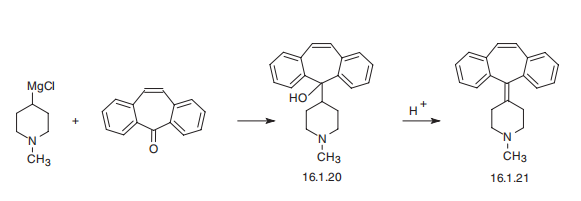
Cyproheptadine, 4-(dibenzo[a,d]cyclohepten-5-ylidene)-1-methylpiperidine (16.1.21), is synthesized by reacting 1-methyl-4-magnesiumchloropiperidine with 5H-dibeno[a,d]cycloheptene-5-one, which forms carbinol (16.1.20), the dehydration of which in an acidic medium leads to the formation of cyproheptadine (16.1.21).
Cyproheptadine Preparation Products And Raw materials
Raw materials
1of2
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | info@tianfuchem.com | China | 21639 | 55 |
| Career Henan Chemica Co | +86-0371-86658258 +8613203830695 | laboratory@coreychem.com | China | 30241 | 58 |
| Hebei Zhanyao Biotechnology Co. Ltd | 15369953316 +8615369953316 | admin@zhanyaobio.com | China | 2123 | 58 |
| Dayang Chem (Hangzhou) Co.,Ltd. | 571-88938639 +8617705817739 | info@dycnchem.com | China | 52849 | 58 |
| Zhejiang J&C Biological Technology Co.,Limited | +1-2135480471 +1-2135480471 | sales@sarms4muscle.com | China | 10473 | 58 |
| Shaanxi Didu New Materials Co. Ltd | +86-89586680 +86-13289823923 | 1026@dideu.com | China | 8670 | 58 |
| PT CHEM GROUP LIMITED | peter68@ptchemgroup.com | China | 35425 | 58 | |
| GIHI CHEMICALS CO.,LIMITED | +8618058761490 | info@gihichemicals.com | China | 49979 | 58 |
| Hebei Miaoyin Technology Co.,Ltd | +86-17367732028 +86-17367732028 | kathy@hbyinsheng.com | China | 3512 | 58 |
| LEAPCHEM CO., LTD. | +86-852-30606658 | market18@leapchem.com | China | 43340 | 58 |
View Lastest Price from Cyproheptadine manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2024-10-24 | Cyproheptadine
129-03-3
|
US $1130.00 / mg | 10g | TargetMol Chemicals Inc. | |||
 |
2021-10-30 | Cyproheptadine
129-03-3
|
US $10.00 / Kg/Bag | 1Kg/Bag | 99% | 20 Tons | Hebei Zhanyao Biotechnology Co. Ltd |
-

- Cyproheptadine
129-03-3
- US $1130.00 / mg
- TargetMol Chemicals Inc.
-

- Cyproheptadine
129-03-3
- US $10.00 / Kg/Bag
- 99%
- Hebei Zhanyao Biotechnology Co. Ltd
129-03-3(Cyproheptadine)Related Search:
1of4