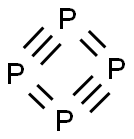
White phosphorus
- CAS No.
- 12185-10-3
- Chemical Name:
- White phosphorus
- Synonyms
- tetraphosphorus;Chebi:35895;Phosphorus (YP);white phosphorus;Phosphorus tetramer;Molecular phosphorus;Tetraatomic phosphorus;Tetrahedro-tetraphosphorus;Phosphorus tetraatomic molecule;White phosphorus ISO 9001:2015 REACH
- CBNumber:
- CB82131283
- Molecular Formula:
- P4
Lewis structure

- Molecular Weight:
- 123.896
- MDL Number:
- MFCD03701484
- MOL File:
- 12185-10-3.mol
| Melting point | 44.1 (0.181mm) [MER06] |
|---|---|
| Boiling point | 277℃ [CRC10] |
| Density | α: 1.83; β. 1.88 [MER06] |
| Water Solubility | 1 part/300,000 parts H2O; 1g/400mL absolute alcohol; 1 g/200mL CHCl2; 1g/40mL benzene [MER06] |
| EWG's Food Scores | 2 |
| FDA UNII | REJ3TH2WED |
| NIST Chemistry Reference | Phosphorus tetramer(12185-10-3) |
| EPA Substance Registry System | Phosphorus (white or yellow) (12185-10-3) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |     GHS02,GHS06,GHS05,GHS09 |
|---|---|
| Signal word | Danger |
| Hazard statements | H250-H300-H314-H330-H400 |
| Precautionary statements | P210-P222-P280-P302+P334-P370+P378-P422-P264-P270-P301+P310-P321-P330-P405-P501-P260-P264-P280-P301+P330+P331-P303+P361+P353-P363-P304+P340-P310-P321-P305+P351+P338-P405-P501-P260-P271-P284-P304+P340-P310-P320-P403+P233-P405-P501-P273-P391-P501 |
White phosphorus price More Price(2)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| American Custom Chemicals Corporation | CHM0293138 | WHITE PHOSPHORUS 95.00% | 12185-10-3 | 5MG | $774.4 | 2021-12-16 | Buy |
| American Custom Chemicals Corporation | CHM0293138 | WHITE PHOSPHORUS 95.00% | 12185-10-3 | 50MG | $1871.1 | 2021-12-16 | Buy |
White phosphorus Chemical Properties,Uses,Production
Description
White phosphorus,also known as yellow phosphorus, is a nonmetallic element that is found in the form of crystals or a wax-like transparent solid. It ignites spontaneously in air at 86°F, which is also its ignition temperature. White phosphorus should be stored under water and away from heat. It is a dangerous fire risk, with a boiling point of 536°F (280°C) and a melting point of 111°F (43°C). White phosphorus is toxic by inhalation and ingestion, and contact with skin produces burns. The TLV is 0.1 mg/m3 of air, and it is insoluble in water, with a specific gravity of 1.82, which is heavier than air. White phosphorus is shipped and stored under water to keep it from contacting air. The four-digit UN identification number is 2447. The NFPA 704 designation is health 4, flammability 4, and reactivity 2. The primary uses are in rodenticides, smoke screens, and analytical chemistry.
Chemical Properties
Phosphorus is a white to yellow, soft, waxy solid with acrid fumes in air. White/yellow phosphorus is either a yellow or colorless, volatile, crystalline solid which darkens when exposed to light and ignites in air to form white fumes and greenish light. It has a garlic-like odor. Usually shipped or stored in water.
Potential Exposure
White or yellow phosphorus is handled away from air so that exposure is usually limited. Phosphorus was at one time used for the production of matches or “lucifers” but has long since been replaced due to its chronic toxicity. It is used in the manufacture of munitions including tracer bullets, pyrotechnics, explosives, smoke bombs; and other incendiary agents; (because it spontaneously catches fire in air) and as a smoke agent (because it produces clouds of irritating white smoke). Phosphorus is used artificial fertilizers; rodenticides, phosphor bronze alloys; semiconductors, Electro-luminescent coating; and chemicals, such as phosphoric and metallic phosphides. RP is used as a choking/pulmonary agent
Shipping
UN1338 Phosphorus, amorphous, Hazard Class: 4.1; Labels: 4.1-Flammable solid. UN1381 Phosphorus, white, dry; under water, in solution; Phosphorus, yellow, dry; yellow, under water; in solution, Hazard Class: 4.2; Labels: 4.2-Spontaneously combustible material, 6.1Poisonous materials. UN2447 Phosphorus white, molten, Hazard Class: 4.2; Labels: 4.2-Spontaneously combustible material, 6.1-Poisonous materials.
Incompatibilities
Phosphorus, a pyrophoric solid, spontaneously ignite on contact with air, producing toxic phosphorus oxide fumes. Reacts with strong bases, releasing toxic phosphine gas. Phosphorus reacts violently with oxidizers, halogens, some metals, nitrites, sulfur, and many other compounds, causing a fire and explosion hazard. White/yellow reacts with air, halogens, halides, sulfur, oxidizers, alkali hydroxides (forming gas); and metals (forming reactive phosphides). Red is a combustible solid. Friction or contact with oxidizers can cause ignition. Incompatible with many other substances. Forms gas and phosphoric acid on contact with moisture. Opened packages of red phosphorus should be stored under inert gas blanket.
Waste Disposal
Controlled incineration followed by alkaline scrubbing and particulate removal equipment.
White phosphorus Preparation Products And Raw materials
Raw materials
Preparation Products
1of6
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Hefei TNJ Chemical Industry Co.,Ltd. | +86-0551-65418679 +86-18949832763 | info@tnjchem.com | China | 2989 | 55 |
| Hubei Jusheng Technology Co.,Ltd. | 18871490254 | linda@hubeijusheng.com | CHINA | 28180 | 58 |
| Hubei xin bonus chemical co. LTD | 86-13657291602 | linda@hubeijusheng.com | CHINA | 22968 | 58 |
| Hefei TNJ Chemical Industry Co.,Ltd. | 0551-65418671 | sales@tnjchem.com | China | 34572 | 58 |
| Shaanxi Dideu Medichem Co. Ltd | +86-029-89586680 +86-18192503167 | 1026@dideu.com | China | 9271 | 58 |
| Protheragen-ING | +16313385890 | info@protheragen-ing.com | United States | 1091 | 58 |
| Chemwill Asia Co.,Ltd. | 86-21-51086038 | chemwill_asia@126.com | CHINA | 23931 | 58 |
| Credit Asia Chemical Co., Ltd. | +86 (21) 61124340 | China | 9767 | 58 | |
| Shanghai Orgchem Co.,Ltd. | +86-21-5877 1921 | info@chemofchina.com | China | 9661 | 55 |
| Shanghai Billion Chemical Chemical Co. Ltd. | 021-58771921 13818686088 | info@chemofchina.com | China | 2989 | 58 |