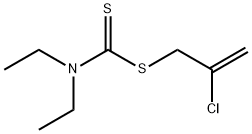
SULFALLATE
- CAS No.
- 95-06-7
- Chemical Name:
- SULFALLATE
- Synonyms
- CDEC;cp4572;cp4742;Vegedex;CDEC(R);CP 4572;cp4,742;Vegadex;CP 4,742;NSC 16085
- CBNumber:
- CB8270999
- Molecular Formula:
- C8H14ClNS2
- Molecular Weight:
- 223.79
- MDL Number:
- MFCD00048530
- MOL File:
- 95-06-7.mol
| Melting point | 25°C |
|---|---|
| Boiling point | bp1 128-130° |
| Density | d25 1.088 |
| refractive index | nD25 1.5822 |
| Flash point | >100 °C |
| storage temp. | APPROX 4°C |
| pka | 0.71±0.50(Predicted) |
| Water Solubility | 0.1g/L(25 ºC) |
| Merck | 13,8996 |
| Stability | Stable. Incompatible with alkalies, strong oxidizing agents. |
| EWG's Food Scores | 3 |
| FDA UNII | 7U7BI7173J |
| Proposition 65 List | Sulfallate |
| IARC | 2B (Vol. 30, Sup 7) 1987 |
| EPA Substance Registry System | Sulfallate (95-06-7) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |    GHS08,GHS07,GHS09 |
|---|---|
| Signal word | Danger |
| Hazard statements | H302-H350-H410 |
| Precautionary statements | P264-P270-P301+P312-P330-P501-P273-P391-P501 |
| Hazard Codes | T,N |
| Risk Statements | 45-22-50/53 |
| Safety Statements | 53-45-60-61 |
| RIDADR | UN3082 9/PG 3 |
| RTECS | EZ5075000 |
| HS Code | 29302000 |
| Toxicity | LD50 orally in rats: 850 mg/kg (Bailey, White) |
SULFALLATE price More Price(4)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Medical Isotopes, Inc. | 42262 | Sulfallate | 95-06-7 | 100mg | $650 | 2021-12-16 | Buy |
| American Custom Chemicals Corporation | PST0000297 | SULFALLATE 95.00% | 95-06-7 | 100MG | $750.75 | 2021-12-16 | Buy |
| American Custom Chemicals Corporation | PST0000297 | SULFALLATE 95.00% | 95-06-7 | 1G | $1963.5 | 2021-12-16 | Buy |
| Medical Isotopes, Inc. | 42262 | Sulfallate | 95-06-7 | 1g | $2200 | 2021-12-16 | Buy |
SULFALLATE Chemical Properties,Uses,Production
Chemical Properties
Sulfallate is an amber liquid.
Uses
Sulfallate is a selective pre-planting or pre-emergence herbicide.
Definition
ChEBI: Sulfallate is a thiocarbonyl compound.
General Description
Amber to dark amber liquid.
Air & Water Reactions
Insoluble in water. Thio and dithiocarbamates slowly decompose in aqueous solution to form carbon disulfide and methylamine or other amines. Such decompositions are accelerated by acids.
Reactivity Profile
SULFALLATE is incompatible with strong oxidizing agents .Also incompatible with alkalis. .
Fire Hazard
SULFALLATE is probably combustible.
Safety Profile
Confirmed carcinogen with experimental carcinogenic data. Moderately toxic by ingestion and skin contact. Mutation data reported. An herbicide. When heated to decomposition it emits very toxic fumes of Cl-, NOx, and SOx. See also ALLYL COMPOUNDS, CARBAMATES, and ESTERS.
Potential Exposure
A dithiocarbamate. The major use for sulfallate in the United States is as a preemergent selective herbicide to control certain annual grasses and broadleaf weeds around vegetable and fruit crops. Sulfallate has also been used for weed control among shrubbery and ornamental plants. Some dithiocarbamates have been used as rubber components
Carcinogenicity
Sulfallate is reasonably anticipated to be a human carcinogen based on sufficient evidence of carcinogenicity from studies in experimental animals.
Shipping
UN2771 Dithiocarbamate and Thiocarbamate pesticides, solid, toxic, Hazard Class: 6.1; Labels: 6.1-Poisonous materials. UN3082 Environmentally hazardous substances, liquid, n.o.s., Hazard class: 9; Labels: 9-Miscellaneous hazardous material, Technical Name Required.
Incompatibilities
Dithiocarbamate esters are combustible. They react violently with powerful oxidizers such as calcium hypochlorite. Poisonous gases are generated by the thermal decomposition of dithiocarbamate compounds, including carbon disulfide, oxides of sulfur, oxides of nitrogen, hydrogen sulfide, ammonia, and methylamine.Thio and dithiocarbamates slowly decompose in aqueous solution to form carbon disulfide and methylamine or other amines. Such decompositions are accelerated by acids. Flammable gases are generated by the combination of dithiocarbamate with aldehydes, nitrides, and hydrides. Dithiocarbamate are incompatible with acids, peroxides, and acid halides.
Waste Disposal
Small amounts may be decomposed by strong oxidizing agent. Large amounts should be incinerated in a unit with effluent gas scrubbing.
SULFALLATE Preparation Products And Raw materials
Raw materials
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Hubei Jusheng Technology Co.,Ltd. | 18871490254 | linda@hubeijusheng.com | CHINA | 28180 | 58 |
| Hubei xin bonus chemical co. LTD | 86-13657291602 | linda@hubeijusheng.com | CHINA | 22968 | 58 |
| Chongqing Chemdad Co., Ltd | +86-023-61398051 +8613650506873 | sales@chemdad.com | China | 39916 | 58 |
| Mainchem Co., Ltd. | +86-0592-6210733 | sale@mainchem.com | China | 32360 | 55 |
| Shaanxi Didu New Materials Co. Ltd | +86-89586680 +86-13289823923 | 1026@dideu.com | China | 9020 | 58 |
| Chemsky (shanghai) International Co.,Ltd | 021-50135380 | shchemsky@sina.com | China | 15421 | 60 |
| Shenzhen Polymeri Biochemical Technology Co., Ltd. | +86-400-002-6226 13028896684 | sales@rrkchem.com | China | 55954 | 58 |
| ShangHai Anpel Co, Ltd. | 18501792038; 18501792038 | shanpel@anpel.com.cn | China | 9614 | 58 |
| TCI (Shanghai) Chemical Trading Co., Ltd. | 021-61109150 | sales@tcisct.com | China | 31163 | 58 |
| Shanghai Bikai Technology Co. , Ltd. | 18516304666 | christina.feng@cpachem-sh.com | China | 3101 | 58 |