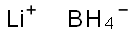Lithium borohydride
- CAS No.
- 16949-15-8
- Chemical Name:
- Lithium borohydride
- Synonyms
- LiBH4;PQHL;Lithium Borohydride in THF;LITHIUM TETRAHYDROBORATE;Lithium borohydride (LBH);lithium borohydride solution;Lithium borohydride2M solution in tetrahydrofuranAcroSeal§3;Lithiumboranat;Lithiumborhydrid;LITHIUM BORANATE
- CBNumber:
- CB8732729
- Molecular Formula:
- BH4Li
- Molecular Weight:
- 21.78
- MDL Number:
- MFCD00011088
- MOL File:
- 16949-15-8.mol
| Melting point | 280 °C |
|---|---|
| Boiling point | 66°C/760mmHg |
| Density | 0.896 g/mL at 25 °C |
| Flash point | −1 °F |
| storage temp. | water-free area |
| solubility | Soluble in ether, THF, and aliphatic aminesSoluble in ether, tetrahydrofuran, aliphatic amines and ethanol. |
| form | Powder |
| Specific Gravity | 0.66 |
| color | White |
| explosive limit | 4.00-75.60%(V) |
| Water Solubility | soluble H2O above pH 7, ether, tetrahydrofuran, aliphatic amines [MER06] |
| Sensitive | Air & Moisture Sensitive |
| Merck | 14,5525 |
| CAS DataBase Reference | 16949-15-8(CAS DataBase Reference) |
| EWG's Food Scores | 1 |
| FDA UNII | 8L87X4S4KP |
| NIST Chemistry Reference | Lithium tetrahydroborate(16949-15-8) |
| EPA Substance Registry System | Borate(1-), tetrahydro-, lithium (16949-15-8) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |    GHS02,GHS05,GHS06 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H260-H301-H314 | |||||||||
| Precautionary statements | P231+P232-P260-P280-P303+P361+P353-P304+P340+P310-P305+P351+P338 | |||||||||
| Hazard Codes | F,T,C,Xn,F+ | |||||||||
| Risk Statements | 14/15-23/24/25-34-20/21/22-11-40-36/37/38-19-67-66-22-12 | |||||||||
| Safety Statements | 26-36/37/39-43-45-36/37-16 | |||||||||
| RIDADR | UN 3399 4.3/PG 1 | |||||||||
| WGK Germany | 2 | |||||||||
| RTECS | ED2725000 | |||||||||
| F | 10-21 | |||||||||
| TSCA | Yes | |||||||||
| HS Code | 2850 00 20 | |||||||||
| HazardClass | 4.3 | |||||||||
| PackingGroup | I | |||||||||
| NFPA 704 |
|
Lithium borohydride Chemical Properties,Uses,Production
Physical Properties
White orthorhombic crystals; density 0.67 g/cm3; decomposes in moist air; melts at 268°C; decomposes at 380°C; reacts with water; dissolves in ether, tetrahydrofuran, and diethylamine; solubility in ether, 25g/L at 25°C.
Uses
Lithium borohydride is used as a strong reducing agent. Its principal applications are in organic syntheses for reducing carbonyl groups such as aldehydes, ketones, and esters. It also is used for selectively reducing a carbonyl group in the presence of a nitrile group. Such selective reduction cannot be achieved with lithium aluminum hydride, which is a much stronger reducing agent. The compound also is used to detect free carbonyl groups in proteins and peptides.
Preparation
Lithium borohydride is prepared by reacting ethyllithium with aluminum borohydride:
C2H5Li + Al(BH4)3→3LiBH4 + Al(C2H5)3
Alternatively, the compound may be obtained by the reaction of ethyllithium with diborane:
C2H5Li + B2H6→LiBH4 + C2H5BH2
Reactions
Lithium borohydride reacts with water with liberation of hydrogen:
LiBH4 + 2H2O → LiBO2 + 4H2
Reaction with methanol yields lithium boromethoxide and hydrogen:
LiBH4 + 2CH3OH → LiB(OCH3)2 + 3H2
Reaction with hydrogen chloride yields diborane, lithium chloride and hydrogen:
2LiBH4 + 2HCl → 2LiCl + B2H6 + 2H2
Reactions with oxidizing agents are violent.
Chemical Properties
WHITE POWDER
Physical properties
White orthorhombic crystals; density 0.67 g/cm3; decomposes in moist air; melts at 268°C; decomposes at 380°C; reacts with water; dissolves in ether, tetrahydrofuran, and diethylamine; solubility in ether, 25g/L at 25°C.
Uses
Lithium borohydride (LiBH4) is a complex hydride with a high hydrogen density. It is a strong reducing agent and an electrode material. It has a high gravimetric (18.4 wt%) and volumetric (121 kg/m3) hydrogen storage capacities. It can also be used in lithium-ion batteries.
Uses
Strong reducing agent. Used to reduce compounds containing ketones, aldehydes, ester carbonyls, acid chlorides, lactones, and epoxidesLithium borohydride is a versatile reducing agent for aldehydes, ketones, nitriles, primary amides, acid chlorides, lactones, epoxides, and esters. It is involved in the preparation of intercalation compounds and nanocomposites. It is used as a precursor for other borohydrides and acts as a catalyst in hydroboration reactions. It is known to be good reversible storage compound for anhydrous ammonia and hydrogen.
Uses
Strong reducing agent. Used in the reduction of Compounds contg ketonic, aldehydic, or ester carbonyls and a nitrile group, where reduction of the carbonyl, but not of the nitrile group, is wanted. In the determination of free carboxyl groups in peptides and proteins; after esterification and acetylation, only the ester groups, and none of the peptide bonds are reduced.
General Description
A white to grayish crystalline powder.
Air & Water Reactions
Likely to ignite when moistened with water [Lab. Gov. Chemist 1965].
Reactivity Profile
Lithium borohydride is a strong reducing agent. Is easily ignited and burns vigorously once ignited. Reacts on contact with water or acids to form hydrogen gas and corrosive products. Reaction with limited amounts of water or moisture may cause ignition after a delay [Gaylord, 1965, p. 22].
Health Hazard
Inhalation or contact with vapors, substance or decomposition products may cause severe injury or death. May produce corrosive solutions on contact with water. Fire will produce irritating, corrosive and/or toxic gases. Runoff from fire control may cause pollution.
Fire Hazard
Produce flammable gases on contact with water. May ignite on contact with water or moist air. Some react vigorously or explosively on contact with water. May be ignited by heat, sparks or flames. May re-ignite after fire is extinguished. Some are transported in highly flammable liquids. Runoff may create fire or explosion hazard.
Safety Profile
Poison by ingestion, inhalation, and skin contact. Flammable; can liberate H2. Incompatible with H20 as moisture on fibers of cellulose or as liquid. See also LITHIUM, BORON COMPOUNDS, and HYDRIDES.
Purification Methods
It is crystallised from Et2O, and pumped free of ether at 90-100o during 2hours [Schaeffer et al. J Am Chem Soc 78 729 1956]. Store it dry as it decomposes slowly in moist air. [Becher in Handbook of Preparative Inorganic Chemistry (Ed. Brauer) Academic Press Vol I p 775 1963.]
Lithium borohydride Preparation Products And Raw materials
Raw materials
Preparation Products
1of2
Related articles
- Advancements in Lithium Borohydride for Enhanced Hydrogen Storage
- Lithium Borohydride is a white solid with multiple crystal forms, soluble in polar solvents. Research aims to enhance its hydr....
- Feb 4,2024
- Lithium tetrahydridoborate:Properties,Production and Uses
- Lithium borohydride is used for the selective reduction of esters, carboxylic acids, amides, and epoxides in preference to oth....
- Oct 21,2023