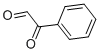
2-(hydroxyimino)-2-phenylacetaldehyde oxime
- CAS No.
- 4589-97-3
- Chemical Name:
- 2-(hydroxyimino)-2-phenylacetaldehyde oxime
- Synonyms
- phenylglyoxime;1-Phenylglyoxal dioxime;α-(Hydroxyimino)benzeneacetaldehyde oxime;2-(hydroxyimino)-2-phenylacetaldehyde oxime;Benzeneacetaldehyde, α-(hydroxyimino)-, oxime
- CBNumber:
- CB9908161
- Molecular Formula:
- C8H8N2O2
- Molecular Weight:
- 164.16132
- MDL Number:
- MOL File:
- 4589-97-3.mol
2-(hydroxyimino)-2-phenylacetaldehyde oxime Chemical Properties,Uses,Production
Preparation
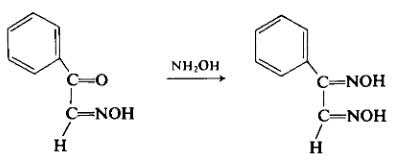
To a solution of 50 gm (0.34 mole) of ω-oximinoacetophenone (ω-isonitrosoacetophenone) in 150 ml of ethanol is added to a solution of 48 gm (0.595 mole) of sodium acetate and 24 gm (0.345 mole) of hydroxylamine hydrochloride in 75 ml of water. The mixture is heated at reflux for 4 hr. After cooling, a large portion of the Solvent is evaporated off under reduced pressure. The mixed crude product, which precipitates, is filtered off, washed with water, and air-dried to afford 51 gm (92%), m.p. 150-158°C.
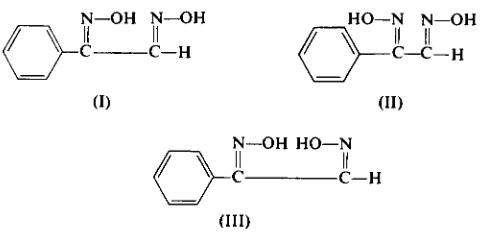
The separation of the three isomers by fractional crystallization procedures depends on the relative ratio of the components in the mixture, the concentration of the components in the solvent, the length of time the solvent-product mixture is heated, etc. It is therefore important that the progress of the crystallization be followed by thin-layer chromatography using freshly prepared tic plates [Merck (Darmstadt) silica gel G is recommended as the coating]. The suggested tic solvent is benzene-ethyl acetate (7:3), detection by iodine vapor; 0.45 Rf for phenyl-amphi-glyoxime (I), 0.40 Rf for phenyl-anti-glyoxime (II), and 0.35 Rf for phenyl-syn-glyoxime(III).
Anti-glyoxime (II), and 0.35 Rf for phenyl-syn-glyoxime(III). Anti-phenyl-amphi-glyoxime (I) is separated from the mixed product by repeated recrystallization from acetone-chloroform (or alcohol-water), m.p. 178-180°C; UV, λmax (95% ethanol) 230 mμ (ε 14,800).
Phenyl-anti-glyoxime (II) is isolated from the mother liquor of the first crystallization in the separation of (I). The residue from the evaporation of this mother liquor is crystallized repeatedly from acetone-chloroform. After sublimation, the properties of the material are as follows: sublimation temperature, 90°C (0.2 mm Hg); m.p. 166-168°C; UV, λmax (95% ethanol) 228 mμ (ε 14,380). Only about 1% of pure isomer is isolated from the starting product.
Phenyl-syn-glyoxime (III) is isolated by crystallizing the starting product from a dilute solution of ethyl acetate, m.p. 168-170°C; UV, λmax (95% ethanol) 252 mμ (ε 12,200).
2-(hydroxyimino)-2-phenylacetaldehyde oxime Preparation Products And Raw materials
Raw materials
Preparation Products
4589-97-3(2-(hydroxyimino)-2-phenylacetaldehyde oxime)Related Search:
1of3