1,1,2-トリフルオロ-2-クロロエテン 化学特性,用途語,生産方法
解説
1-クロロ-1,2,2-トリフルオロエテン.1,2-ジクロロ-1,1,2-トリフルオロエタンの脱塩化水素,あるいはトリクロロトリフルオロエタンの亜鉛または水素による脱塩素により製造する.融点-168.1 ℃,沸点-27.9 ℃.臨界温度105.8 ℃.臨界圧41.4 kg cm-2.燃焼範囲16~34体積%(空気中).レドックス触媒法やエマルション重合による高重合体は,形成加工性がテフロンよりすぐれたフッ素樹脂として有用であり,エテンとの共重合体は,耐熱,耐衝撃性にすぐれている.低重合体は,潤滑油,グリースとして用いられる.取り扱いには耐爆設備を使用する.
用途
レドックス触媒法エマルジョン重合による高重合体は成形加工性がテフロンよりすぐれたフッ素樹脂として有用、低重合体は潤滑油、グリース、ワックスとして用いられる。エチレンとの交互共重合体は耐熱、耐ストレスクラッキング性、耐衝撃性がすぐれたフッ素樹脂となる。
化学的特性
Colorless gas; faint ethereal odor.
Decomposes in water.
使用
Intermediate, monomer for chlorotrifluoroethylene resins.
製造方法
Chlorotrifluoroethylene is prepared from ethylene by the following route:
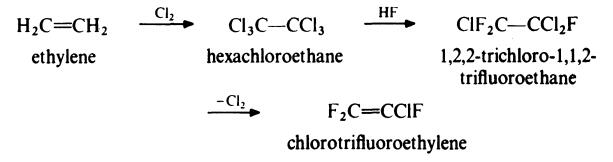
Ethylene is treated with an excess of chlorine at 300-350??C in the presence of
activated charcoal to give hexachloroethane. This product is then treated
with hydrogen fluoride in the presence of antimony pentachloride to yield
trichlorotrifluoroethane. Dechlorination in the liquid phase with zinc dust
and ethanol results in the formation of chlorotrifluoroethylene which is
washed with water to remove alcohol, dried and distilled under pressure.
一般的な説明
Chlorotrifluoroethylene is a colorless gas with a faint ethereal odor. Chlorotrifluoroethylene is shipped as a liquefied gas under its vapor pressure. Chlorotrifluoroethylene is very toxic by inhalation and is easily ignited. The vapors are heavier than air and a flame can flash back to the source of leak very easily. This leak can either be a liquid or vapor leak. The vapors can asphyxiate by the displacement of air. Contact with the liquid can cause frostbite. Under prolonged exposure to intense heat or fire the containers may violently rupture and rocket, or the material may polymerize with possible container rupture.
空気と水の反応
Highly flammable.
反応プロフィール
Halogenated aliphatic compounds, such as Chlorotrifluoroethylene, are moderately or very reactive. Reactivity generally decreases with increased degree of substitution of halogen for hydrogen atoms. Low molecular weight haloalkanes are highly flammable and can react with some metals to form dangerous products. Materials in this group are incompatible with strong oxidizing and reducing agents. Also, they are incompatible with many amines; nitrides; azo/diazo compounds; alkali metals; strong oxidizers such as chlorine perchlorate, oxygen, bromine; and epoxides.
危険性
Dangerous fire risk. Flammable limits in
air 8.4–38.7%.
健康ハザード
Inhalation causes dizziness, nausea, vomiting; liver and kidney injury may develop after several hours and cause jaundice and necrosis of the kidney. Contact with liquid causes frostbite of eyes and possibly of skin.
安全性プロファイル
Poison by ingestion and
intraperitoneal routes. Moderately toxic by
inhalation. Very dangerous fire hazard when
exposed to heat, flames (sparks), or
oxidizers. To fight fire, stop flow of gas.
Violent reaction when mixed with (Brz +
02) or (CIF3 + water). Potentially explosive
polymerization reaction with ethylene.
Incompatible with 1,l -dchloroethylene;
oxygen. When heated to decomposition it
emits toxic fumes of Fand Cl-. See also
CHLORINATED HYDROCARBONS,
ALIPHATIC; and FLUORIDES.
純化方法
Scrub it with 10% KOH solution, then 10% H2SO4 solution to remove inhibitors, dry and pass it through silica gel. It is stabilized with ~1% tributylamine. Use brass equipment. [Beilstein 1 III 646.] TOXIC GAS.
1,1,2-トリフルオロ-2-クロロエテン 上流と下流の製品情報
原材料
亜鉛 (粉末)
塩化亜鉛
ナトリウム チオサルフェト 五水和物
1,1,2-トリフルオロトリクロロエタン
Silane, trichloro(2,2-dichloro-1,1,2-trifluoroethyl)-
1-bromo-1,2,2-trifluoro-1,2-chloroethane
1,2-ジクロロ-1,2,2-トリフルオロエチルヨージド
ジクロロテトラフルオロエタン
1,1-ジクロロ-1,2-ジフルオロエタン
1,1,2-トリフルオロエテン
1,2-ジブロモ-1-クロロ-1,2,2-トリフルオロエタン
準備製品