O-클로로페놀 C화학적 특성, 용도, 생산
화학적 성질
2-Chlorophenol is a colourless to light brown liquid, All isomers have a characteristic odor. Slightly soluble in water, soluble in ethanol, ether and alkali solution. It is used to make dyes and other chemicals, and as a disinfectant, bactericide, and germicide.
물리적 성질
Pale amber liquid with a slight phenolic, floral, or musty-type odor. At 40 °C, the average and
lowest odor concentrations detected were 0.36 and 0.088 μg/L, respectively. At 25 °C, the average
taste threshold concentration and the lowest concentration at which a taste was detected were 0.97
and 0.94 μg/L, respectively (Young et al., 1996).
제조 방법
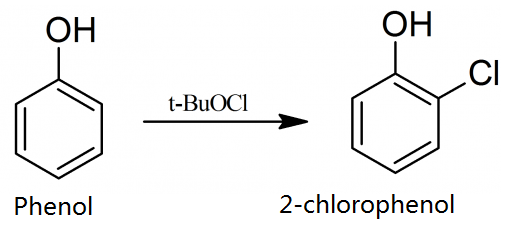
2-chlorophenol is produced in 22% yields from the reaction of chlorine monoxide with phenol in carbon tetrachloride: 0.5 mole of tert-butyl hypochlorite is dropped, with stirring, into a solution or suspension of the 0.5 mole of phenol in 150-300 ml of carbon tetrachloride, the temperature being allowed to rise to the boiling point of the solvent. The mixture is then boiled under reflux for a further 2 hours, the carbon tetrachloride and the tert-butyl alcohol are distilled off, and the residue is fractionated collecting fraction boiling at 174.9 °C.
주요 응용
2-chlorophenol is used in medicine, pesticides, dyes and organic synthesis. It is an intermediate in the production of the pesticides Profenofos and Isoprocarb. It is also utilized in the manufacture of dye-stulfs and in the process for extracting sulphur and nitrogen compounds from coal. 2-chlorophenol functions as a reagent in the preparation of chlorophenyl phosphorodichloridothioate as potential synthon for oligonucleotide phosphorothioates.
정의
ChEBI: 2-chlorophenol is a monochlorophenol and a 2-halophenol.
일반 설명
2-chlorophenol appears as a colorless to amber liquid with an unpleasant, penetrating odor. Density 1.265 g / cm3. Sinks in water and slowly dissolves. Freezing point 7°C (46°F). Boiling point 175°C (347°F).
공기와 물의 반응
Very soluble in water
반응 프로필
2-Chlorophenol is a weak acid. Neutralizes bases in exothermic reactions. Incompatible with oxidizing agents. Incompatible with acid chlorides and acid anhydrides. Forms ethers, esters and salts with metals and amines .
위험도
Toxic by skin absorption, inhalation, or
ingestion. Strong irritant to tissue.
건강위험
Poisonous; may be fatal if inhaled, swallowed or absorbed through skin. Irritating to skin and eyes; direct contact may cause burns. Rats receiving lethal doses via oral, subcutaneous or intraperitoneal routes displayed similar symptoms: restlessness, increased breathing rate and motor weakness followed by tremors, chronic convulsions, dyspnea, coma and death.
Safety Profile
Poison by ingestion,
intraperitoneal, and intravenous routes.
Experimental reproductive effects.
Questionable carcinogen with experimental
tumorigenic data. Mutation data reported.
Flammable liquid when exposed to heat,
flame, or oxidizers. To fight fire, use
alcohol foam. When heated to
decomposition it emits toxic fumes of Cl-.
See also CHLOROPHENOLS and
CHLORIDES.
잠재적 노출
Monochlorophenols are used in the
manufacture of fungicides, slimicides, bactericides, pesticides, herbicides, disinfectants, wood and glue preservatives; in the production of phenolic resins; in the extraction
of certain minerals from coal; as a denaturant for ethanol;
as an antiseptic; as a disinfectant, and others.
운송 방법
UN 2020 (solid); UN2021 (liquid) Chlorophenols, solid and liquid, Hazard Class: 6.1; Labels:
6.1-Poisonous materials.
Purification Methods
Pass 2-chlorophenol at least twice through a gas chromatography column. It has also been purified by fractional distillation. [Beilstein 6 IV 782.]
비 호환성
May form explosive mixture with air.
Contact with oxidizing agents can cause fire and explosion
hazard. Heat produces hydrogen chloride and chlorine.
Corrosive to aluminum, copper and other chemically active
metals.
폐기물 처리
Incinerate in admixture with
flammable solvent in furnace equipped with afterburner
and scrubber.
O-클로로페놀 준비 용품 및 원자재
원자재
준비 용품