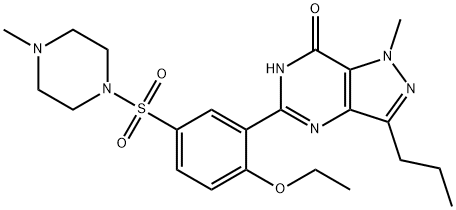
실데나필
|
|
실데나필 속성
- 녹는점
- 187-189°C
- 밀도
- 1.39±0.1 g/cm3(Predicted)
- 인화점
- 9℃
- 저장 조건
- Sealed in dry,Store in freezer, under -20°C
- 용해도
- DMSO(약간 용해됨), 메탄올(약간 용해됨)
- 물리적 상태
- 고체
- 물리적 상태
- 단단한 모양
- 산도 계수 (pKa)
- pKa 8.7 (Uncertain)
- 색상
- 흰색에서 황백색까지
- InChIKey
- BNRNXUUZRGQAQC-UHFFFAOYSA-N
- CAS 데이터베이스
- 139755-83-2(CAS DataBase Reference)
안전
- 위험 및 안전 성명
- 위험 및 사전주의 사항 (GHS)
| 위험품 표기 | Xi,T,F | ||
|---|---|---|---|
| 위험 카페고리 넘버 | 36/37/38-39/23/24/25-23/24/25-11 | ||
| 안전지침서 | 22-24/25-36-26-45-36/37-16-7 | ||
| 유엔번호(UN No.) | UN1230 - class 3 - PG 2 - Methanol, solution | ||
| WGK 독일 | 1 | ||
| HS 번호 | 38220090 | ||
| 유해 물질 데이터 | 139755-83-2(Hazardous Substances Data) |
실데나필 C화학적 특성, 용도, 생산
개요
1998년 화이자(Pfizer Inc.) 제약에서 개발하여 비아그라(Viagra)라는 상표명으로 출시된 남성의 발기부전 치료제. 보통 sildenafil citrate(구연산 실데나필) 형태로 사용된다. 본디 심장 질환 치료를 목적으로 개발된 약이었으나 임상 실험 과정에서 정작 심장 질환 치료 효과는 그저 그래서 사장될 뻔 했다가 약물을 처방받은 환자에서 발기가 일어나는 부작용이 발견되어 이후 발기부전 치료제로 쓰이게 되었다.용도
은 PDE5를 선택적으로 억제하여, 음경해면체내의 cGMP의 양을 증가시킴니다. 이로 인해 평활근이 이완되어 음경해면체내의 혈액이 유입됨으로서 발기부전의 치료에 효과를 보입니다.개요
Sildenafil was launched as Viagra in the US for the treatment of organic orland psychological male erectile dysfunction (ED). It is an orally bioavailable pyrazolopyrimidinone derivative structurally related to zaprinast, with vasodilating and potential anti-inflammatory activities. Upon oral administration, sildenafil selectively targets and inhibits cyclic guanosine monophosphate (cGMP)-specific phosphodiesterase type 5 (PDE5), thereby inhibiting the PDE5-mediated degradation of cGMP found in smooth muscle and increasing cGMP availability. This results in prolonged smooth muscle relaxation in the corpus cavernosum of the penis, thereby causing vasodilation, blood engorgement and a prolonged penile erection.화학적 성질
Sildenafil citrate is a white to off-white crystalline powder soluble in DMF, acetic acid and slightly soluble in methanol. Solubility of sildenafil citrate is pH dependent and it decreases with increase of pH. pH ranges between 3.7 and 3.8 and the pKa from 8.2 to 9.6.용도
Sildenafil is a phosphodiesterase-5 (PDE5) inhibitor. It is indicated for the treatment of erectile dysfunction (ED). Sildenafil is an orally active selective type 5 cGMP phosphodiesterase inhibitor.제조 방법
The first synthetic route of sildenafil accomplished the preparation of its pyrazole derivative from ethyl 3-butyrylpyruvate and hydrazine hydrate in acetic acid, followed by the selective N-methylation of the pyrazole ring with dimethyl sulfate. The carboxylic acid was obtained after alkaline hydrolysis was subjected to nitration, followed by treatment with concentrated ammonium hydroxide solution to sequentially deliver the corresponding carboxamide derivative. The nitro group of the mentioned carboxamide derivative was then reduced to an amino group by stannous chloride/hydrochloric acid in ethanol, leading to the formation of the main 4-aminopyrazole structure. Mild amidation of the aminopyrazole derivative by the appropriate benzoyl chloride was performed, followed by cyclization mediated by hydrogen peroxide under basic environment which led to the formation of pyrimidinone heterocycle ring. Chloro-suIphonylation of pyrimidinone derivative imposed selectively on the 50 position of the phenyl ring, led to the aroyl sulfonyl chloride derivative which was then coupled with N-methylpiperazine to afford sildenafil.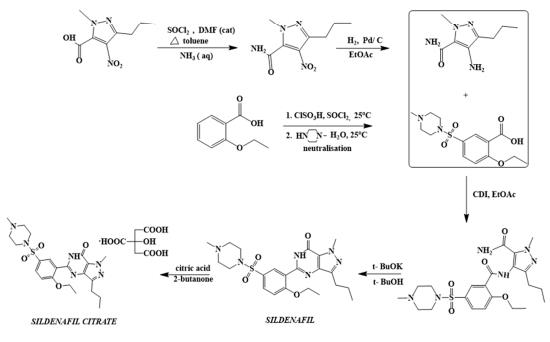
synthesis of sildenafil
정의
ChEBI: Sildenafil is a pyrazolo[4,3-d]pyrimidin-7-one having a methyl substituent at the 1-position, a propyl substituent at the 3-position and a 2-ethoxy-5-[(4-methylpiperazin-1-yl)sulfonyl]phenyl group at the 5-position. It has a role as a vasodilator agent and an EC 3.1.4.35 (3',5'-cyclic-GMP phosphodiesterase) inhibitor. It is a pyrazolopyrimidine, a member of piperazines and a sulfonamide.Indications
Sildenafil (Viagra) is a selective inhibitor of PD-5, an enzyme that inactivates cGMP. Vardenifil (Levitra) is a particularly effective inhibitor of PD-5. It has a shorter onset of action and can be used in smaller doses than sildenafil. Other drugs used in the treatment of ED exert their effects through other biochemical pathways, both central and peripheral.Mechanism of action
Sildenafil is readily absorbed after oral administration and reaches peak plasma levels after about an hour. It undergoes hepatic metabolism and has a terminal half-life of about 4 hours.An initial dose of 50 mg is taken about an hour prior to sexual activity to induce penile erection.Clinical Use
Sildenafil is a selective inhibitor of cGMP-specific PD-5 and therefore inhibits the degradation of cGMP. PD-5, the predominant type in the corpus cavernosum, also is present in other tissues (e.g., lungs, platelets, and eye). The selective inhibition of this enzyme facilitates the release of nitric oxide and smooth muscle relaxation of the corpus cavernosa. Sildenafil enhances erection by augmenting nitric oxide–mediated relaxation pathways. It has been suggested that sildenafil’s mechanism of action is due to cross-talk between cGMP- and cAMPdependent transduction pathways within the cavernous muscles.부작용
Orally administered sildenafil is an effective and well-tolerated treatment for men with ED, including those with diabetes mellitus. It has also been used for so-called salvage therapy in men who do not respond to intracorporeal injections of other agents. Headache is a common side effect, as are flushing and rhinitis.More serious side effects include definite or suspected myocardial infarctions and cardiac arrest.효소 저해제
Sildenafil is rapidly absorbed and peaks in concentration (127–560 ng/mL) after 0.5 to 2.0 hours, displaying a half-life of 3 to 4 hours for the full therapeutic dose (25–100 mg). It is 96% bound to plasma proteins and is metabolized by the liver CYP3A4. The metabolite N-desmethylsildenafil possesses approximately 50% of the activity of the parent molecule.신진 대사
In vitro metabolism studies for sildenafil have shown that the primary metabolite, N-desmethylsildenafil, and the minor metabolite, oxidative opening of the piperazine ring, are mediated by CYP3A4, CYP2C9, CYP2C19, and CYP2D6. The estimated relative contributions to clearance were 79% for CYP3A4, 20% for CYP2C9, and less than 2% for CYP2C19 and CYP2D6. These results demonstrate that CYP3A4 is the primary cytochrome mediating N-demethylation and that drugs inhibiting CYP3A4 likely impair sildenafil biotransformation and clearance. The pharmacokinetics of radiolabeled sildenafil were consistent with rapid absorption, first-pass metabolism, and primarily fecal elimination of N-demethylated metabolites. The absorption of sildenafil following oral administration was rapid (~92%), whereas the oral bioavailability was approximately 38% as a result of first-pass metabolism.실데나필 준비 용품 및 원자재
원자재
암모니아수
1-사이클로프로필-6,7-디플루오로-1,4-디하이드로-8-메톡시-4-옥소-3-퀴놀린카르복실산에틸에스테르
3-프로필-1H-피라졸
Metaclazepam
질산
황산마그네슘
탄산나트륨(경회)
염산
과산화 수소
수산화나트륨
에틸3-n-프로필피라졸-5-카르복실레이트
4-피페콜린
디메틸설페이트
2-Ethoxybenzoyl chloride
염화 제일석
염화 티오닐
트리에틸아민
4-다이메틸아미노피리딘