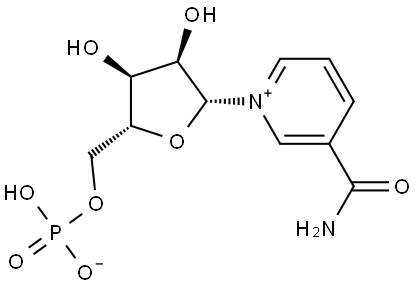
니코틴아마이드모노뉴클레오타이드
|
|
니코틴아마이드모노뉴클레오타이드 속성
- 녹는점
- 166 °C(dec.)
- 저장 조건
- -20°C
- 용해도
- DMSO(약간 용해됨, 가열), 메탄올(약간 용해됨), 물(약간 용해됨)
- 물리적 상태
- 고체
- 물리적 상태
- 단단한 모양
- 색상
- 흰색에서 노란색으로
- Merck
- 13,6697
- BRN
- 3570187
- 안정성
- 흡습성이 매우 높음
- InChI
- InChI=1/C11H15N2O8P/c12-10(16)6-2-1-3-13(4-6)11-9(15)8(14)7(21-11)5-20-22(17,18)19/h1-4,7-9,11,14-15H,5H2,(H3-,12,16,17,18,19)/t7-,8-,9-,11-/s3
- InChIKey
- DAYLJWODMCOQEW-TURQNECASA-N
- SMILES
- O[C@@H]1[C@@H]([C@@H](COP(O)([O-])=O)O[C@H]1[N+]1=CC=CC(C(=O)N)=C1)O |&1:1,2,3,11,r|
안전
- 위험 및 안전 성명
- 위험 및 사전주의 사항 (GHS)
| WGK 독일 | 3 | ||
|---|---|---|---|
| F 고인화성물질 | 8-10-21 | ||
| HS 번호 | 2934999090 |
| 그림문자(GHS): |

|
||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 신호 어: | Warning | ||||||||||||||||||||||||||||
| 유해·위험 문구: |
|
||||||||||||||||||||||||||||
| 예방조치문구: |
|
니코틴아마이드모노뉴클레오타이드 C화학적 특성, 용도, 생산
용도
베타 - 니코틴 아미드 모노 뉴클레오타이드 그것은 인간의 세포 에너지 생산에 중요한 역할을합니다. 심혈관 질환, 뇌 혈관 질환, 호흡기 질환, 제 1 형 당뇨병 및 염증성 면역 질환의 치료에있어 약리학 적 효과의 측면으로는 세포 재생, 노화 방지 등과 같은 세포 에너지 전환에 중요한 코엔자임 니코틴 아미드 아데닌 디 뉴클레오티드의 합성에 참여합니다. 그것은 광범위한 적용 가능성을 가지고 있습니다.개요
Nicotinamide mononucleotide (NMN), a product of the NAMPT reaction and a key NAD+ intermediate, ameliorates glucose intolerance by restoring NAD+ levels in HFD-induced T2D mice. NMN also enhances hepatic insulin sensitivity and restores gene expression related to oxidative stress, inflammatory response, and circadian rhythm, partly through SIRT1 activation. NMN is used for studying binding motifs within RNA aptamers and ribozyme activation processes involving β-nicotinamide mononucleotide (β-NMN)-activated RNA fragments.β-Nicotinamide mononucleotide (β-NMN) is an intermediate in the nicotinamide phosphoribosyltransferase (NAMPT)-catalyzed biosynthesis of nicotinamide adenine dinucleotide (NAD+). NAMPT mediates the condensation of nicotinamide with 5-phosphoribosyl-1-pyrophosphate to produce β-NMN. β-NMN adenyltransferase subsequently converts β-NMN to NAD+.
화학적 성질
White to Yellowish lyophilized powder용도
β-Nicotinamide mononucleotide (NMN) is used to study binding motifs within RNA aptamers and ribozyme activation processes involving β-nicotinamide mononucleotide (β-NMN)-activated RNA fragments. NMN is a nucleotide derived from ribose and nicotinamide. Niacinamide (nicotinamide,) is a derivative of vitamin B3, also known as niacin.) As a biochemical precursor of NAD+, it may be useful in the prevention of pellagra.β-Nicotinamide mononucleotide is an intermediate in the biosynthesis of nicotinamide adenine dinucleotide (NAD+). Nicotinamide phosphoribosyltransferase (Nampt) catalyzes the condensation of nicotinamide with 5-phosphoribosyl-1-pyrophosphate to generate β-NMN, which is subsequently converted to NAD+ by β-NMN adenyltransferase.At 50-100 μM, β-NMN has been used to enhance NAD biosynthesis and glucose-stimulated insulin secretion in a Nampt+/- mouse model of metabolic disease, demonstrating a role for Nampt in β cell function.Furthermore, at 500 mg/kg/day, it has been shown to ameliorate glucose intolerance in high-fat diet-induced type 2 diabetes mice by restoring NAD+ levels.
정의
ChEBI: β-Nicotinamide Mononucleotide is a condensation product of nicotinamide and ribose 5-phosphate, in which the nitrogen of nicotinamide is linked to the (β) c-l of the ribose. NMN zwitterion is a nicotinamide mononucleotide. It has a role as an Escherichia coli metabolite and a mouse metabolite. It is a conjugate base of a NMN(+). It is a conjugate acid of a NMN(-).주요 응용
β-Nicotinamide mononucleotide (NMN) is a product of the extracellular Nicotinamide phosphoribosyltransferase (eNAMPT) reaction and a key NAD+ intermediate. It ameliorates glucose intolerance by restoring NAD+ levels in HFD-induced T2D mice . It also enhances hepatic insulin sensitivity and restores gene expression related to oxidative stress, inflammatory response, and circadian rhythm, partly through SIRT1 activation. It is used to study binding motifs within RNA aptamers and ribozyme activation processes involving β-nicotinamide mononucleotide (β-NMN)-activated RNA fragments.제조 방법
β-Nicotinamide mononucleotide is a NAD+intermediate. In recent years, the relation of NAD+metabolism and aging-associated disease is attracting attention from various research fields.Synthesis of β-nicotinamide mononucleotide (NMN)
A solution of NAD (3.5 g, 5.28 mmol) and ZrCl4(6.15 g, 26.4 mmol) in 500 ml water was stirred at 50°C for 30 min. The hydrolysis was monitored by TLC (SiO2EtOH/ 1 M NH4Ac [7 : 3]). The reaction was quenched with 245mL of a 0.5 M solution of Na3PO4. After adjusting to pH 7 with a 2 M solution of HCl, a white precipitate was formed. The suspension was centrifuged 8 min,1,000rpm, the supernatant was collected and the pellet was washed two times with 200 mL water. The combined supernatants wereconcentrated to 1/3 of its volume on a rotary evaporator. The remaining solution was purified with a column filled with Dowex 50WX8 (100-200 mesh, H+-Form, column-material: 2.5 x 30 cm). The column was loaded with 1.5 L5 % HCl and equilibrated with1.5L millipore water until pH 5 was reached. 100 mL of the concentrated solution was loaded on the ion exchange column and eluted with Milliporewater. The first cleavage product eluted was NMN (615 mg, 1.84 mmol,yield:35 %) and yielded a colorless solid after evaporation of the solvent, followed by AMP.
1H NMR (500MHz, D2O)δ: 9.48 (s, 1 H), 9.31 (d,J= 6.2 Hz, 1 H), 9.00 (d,J= 8.2 Hz, 1 H), 8.32 (dd,J= 8.2, 6.2 Hz, 1 H), 6.24 (d,J=5.4 Hz, 1 H), 4.68-4.64 (m, 1 H), 4.58 (t, 1 H), 4.48-4.45 (m, 1 H), 4.36–4.14 (m,J= 12.0, 2 H).
13C NMR (75 MHz, d2o) δ: 165.50, 145.65, 142.15, 139.53, 133.62, 128.19, 99.65, 87.18, 87.06, 77.42, 70.71, 63.89, 63.82.
31P NMR (202 MHz, D2O)δ:-0.03
benefits
As one of the primary precursors of NAD+ and intermediaries in NAD+ biosynthesis, NMN is as essential as NAD+ in the body’s proper functioning of cells. NAD helps cells regulate a number of essential functions that help keep your cells running smoothly, including:energy metabolism, DNA repair, gene expression, and cellular stress responses.일반 설명
β-Nicotinamide mononucleotide (β-NMN) is an intermediate in the nicotinamide phosphoribosyltransferase (NAMPT)-catalyzed biosynthesis of nicotinamide adenine dinucleotide (NAD+). NAMPT mediates the condensation of nicotinamide with 5-phosphoribosyl-1-pyrophosphate to produce β-NMN. β-NMN adenyltransferase subsequently converts β-NMN to NAD+.Biochem/physiol Actions
In light of metabolic control mechanisms and many reports on β-Nicotinamide Mononucleotide (β-NMN), β-NMN is likely to be more effective as an NAD+ precursor than Nam. Research has compared the blood total NAD (NAD++NADH) and urinary excretion amounts of NAD+ catabolites in β-NMN- and Nam-administered rats. The concentration of blood total NAD and liver total NAD showed no significant differences among the three groups. However, when examining the kinetics of the urinary excretion, the urinary excretion of the SUM was lower in the β-NMN group than in the Nam group at 3-6 h after the administration. Moreover, the percentage of the urinary SUM was much lower in the β-NMN group than in the Nam group at 3-6 h. This result suggests that β-NMN is retained in the body for longer than Nam is. In addition, this result means that β-NMN has a higher turnover of salvage biosynthesis of NAD+ than Nam does. The resulting phenomenon accelerates the turnover of salvage biosynthesis of NAD+, which activates the SIRT1 reaction because SIRT1 (histone deacetylase) needs NAD+. Deacetylated histone molecules induce DNA silencing, contributing to anti-aging and longevity. When β-NMN is intraperitoneally injected, β-NMN is dephosphorylated in the blood to form Nam riboside (NR), which is then transported into the cells. NR is re-phosphorylated to form β-NMN, which is then synthesized to NAD+ in the nucleus. In contrast to Nam, which is controlled at the Nam→β-NMN reaction, the β-NMN biosynthesis pathway is not regulated[1].Purification Methods
Purify NMN by passage through a column of Dowex-1 (Clform) and washing with H2O until no absorbance is observed at 260 nm. The tubes containing NMN are pooled, adjusted to pH 5.5-6 and evaporated in vacuo to a small volume. This is adjusted to pH 3 with dilute HNO3 in an ice-bath and treated with 20volumes of Me2CO at 0-5o. The heavy white precipitate is collected by centrifugation at 0o. It is best stored wet and frozen or it can be dried to give a gummy residue. It has max 266nm ( 4,600) and min 249nm ( 3600) at pH 7.0 (i.e. no absorption at 340nm). It can be estimated by reaction with CNor hydrosulfite which form the 4-adducts (equivalent to NADH) which have UV max 340nm ( 6,200). Thus after reaction, an OD340 of one is obtained from a 0.1612mM solution in a 1cm path cuvette. [Plaut & Plaut Biochemical Preparations 5 56 1957, Maplan & Stolzenbach Methods Enzymol 3 899 1957, Kaplan et al. J Am Chem Soc 77 815 1955, Beilstein 22/2 V 168.]니코틴아마이드모노뉴클레오타이드 준비 용품 및 원자재
원자재
준비 용품
니코틴아마이드모노뉴클레오타이드 공급 업체
글로벌( 807)공급 업체
| 공급자 | 전화 | 이메일 | 국가 | 제품 수 | 이점 |
|---|---|---|---|---|---|
| ZhenYiBio Technology Inc | +8615309206328 |
alexxue@zhenyibio.com | China | 299 | 58 |
| Shanghai Longyu Biotechnology Co., Ltd. | +8615821988213 |
info@longyupharma.com | China | 2531 | 58 |
| Guangzhou TongYi biochemistry technology Co.,LTD | +8613073028829 |
mack@tongyon.com | China | 2996 | 58 |
| Sinoway Industrial co., ltd. | 0592-5800732; +8613806035118 |
xie@china-sinoway.com | China | 992 | 58 |
| Maintain Biotech Co., Ltd. | +86-75589664337 +86-15112661142 |
cami@maintain-bio.com | China | 66 | 58 |
| GIHI CHEMICALS CO.,LIMITED | +8618058761490 |
info@gihichemicals.com | China | 50000 | 58 |
| Hangzhou Zelixir Biotech Co., Ltd. | +8618867646786 |
neal.chen@zelixir.com | China | 230 | 58 |
| Chongqing Zhihe Biopharmaceutical Co., Ltd. | +86-18580541567 +86-17782035140 |
sales@zhswyy.com | China | 326 | 58 |
| Shanghai Affida new material science and technology center | +undefined15081010295 |
admin@oudaxin.com | China | 395 | 58 |
| Hebei Mojin Biotechnology Co., Ltd | +8613288715578 |
sales@hbmojin.com | China | 12470 | 58 |
니코틴아마이드모노뉴클레오타이드 관련 검색:
니코틴아미드아데닌 디뉴클레오티드 니코틴아마이드모노뉴클레오타이드
NADP sodium salt
Nicotinamide
Nicotinamide riboside chloride
Nicotinamide riboside
Nicotinic acid
PYRIDINE-2-CARBOXAMIDE
Nicotinamide-N-oxide
Isonicotinamide
3-Cyanopyridine
Inositol
GPI choline
Pro-xylane
β-nicotinamide mononucleotide, reduced form, disodium salt(NMNH)
2,2'-Anhydro-1-beta-D-arabinofuranosylcytosine hydrochloride
NICOTINIC ACID MONONUCLEOTIDE
4-Aminobutyric acid