트라이메틸아민(트리메틸아민)
|
|
트라이메틸아민(트리메틸아민) 속성
- 녹는점
- -117 °C (lit.)
- 끓는 점
- 3-4 °C (lit.)
- 밀도
- 0.63 g/mL at 20 °C (lit.)
- 증기 밀도
- 2.09 (vs air)
- 증기압
- 430 mm Hg ( 25 °C)
- 굴절률
- n
20/D 1.357
- 인화점
- 38 °F
- 저장 조건
- Store at +5°C to +30°C.
- 용해도
- 물에 매우 잘 녹고, 알코올, 에테르, 벤젠, 톨루엔, 자일렌, 에틸벤젠, 클로로포름에 약간 녹습니다. 최대 허용 농도: TLV 10ppm(24mg/m3) 및 STEL 15ppm(36mg) /m3) (ACGIH 1986)
- 물리적 상태
- 액체
- 산도 계수 (pKa)
- pKb (25°): 4.13
- 색상
- 무색의
- 수소이온지수(pH)
- a strong base (pH 9.8)
- 냄새
- 썩어가는 생선 냄새, 썩은 달걀 냄새, 쓰레기 냄새, 소변 냄새가 납니다.
- 폭발한계
- 11.6%
- ?? ??
- 비린내
- Odor Threshold
- 0.000032ppm
- 수용성
- 물에 불용성, 8.9e+005mg/L.
- 어는점
- -117.1℃
- 감도
- Hygroscopic
- Merck
- 14,9710
- JECFA Number
- 1610
- BRN
- 956566
- 노출 한도
- ACGIH: TWA 50 ppm; STEL 100 ppm (Skin)
OSHA: TWA 200 ppm(590 mg/m3)
NIOSH: IDLH 2000 ppm; TWA 200 ppm(590 mg/m3); STEL 250 ppm(735 mg/m3)
- Dielectric constant
- 2.9(4℃)
- 안정성
- Stable. Incompatible with a wide variety of materials, including bases, acids, oxidizing agents, brass, zinc, magnesium, aluminium, mercury, mercury oxides, acid chlorides, acid anhydrides. Hygroscopic. Highly flammable. Readily forms explosive mixtures with air.
- InChIKey
- GETQZCLCWQTVFV-UHFFFAOYSA-N
- LogP
- 0.06
- CAS 데이터베이스
- 75-50-3(CAS DataBase Reference)
안전
- 위험 및 안전 성명
- 위험 및 사전주의 사항 (GHS)
| 위험품 표기 | Xi,C,F+,Xn,F | ||
|---|---|---|---|
| 위험 카페고리 넘버 | 36/37/38-34-20/22-12-41-37/38-20-11 | ||
| 안전지침서 | 26-36-45-36/37/39-16-29-39-3 | ||
| 유엔번호(UN No.) | UN 2924 3/PG 2 | ||
| WGK 독일 | 1 | ||
| RTECS 번호 | YH2700000 | ||
| F 고인화성물질 | 3-10 | ||
| 자연 발화 온도 | 374 °F | ||
| TSCA | Yes | ||
| DOT ClassificationII | 2.1 (Flammable gas) | ||
| 위험 등급 | 3 | ||
| 포장분류 | II | ||
| HS 번호 | 29211100 | ||
| 유해 물질 데이터 | 75-50-3(Hazardous Substances Data) | ||
| 기존화학 물질 | KE-11508 | ||
| 사고대비 물질 필터링 | 13 |
트라이메틸아민(트리메틸아민) C화학적 특성, 용도, 생산
물성
암모니아에 있는 세 개의 수소가 모두 메틸기로 바뀐 삼차 아민. 썩은 고기 냄새가 나는 기체로 물ㆍ알코올ㆍ벤젠 따위에 녹으며, 유기 합성ㆍ용도
소독제 원료 따위로 쓰인다.화학적 성질
Trimethylamine is compressed gas or liquid. Flammable gas. Shipped as a compressed gas, it may be present in an aqueous solution. It has a strong, fishy, ammoniacal odor. The Odor Threshold is 0.00011-0.87 ppm. Warning: The Odor Threshold range is so broad that odor alone should not be used as a warning of potentially hazardous exposures.물리적 성질
Trimethylamine has a pungent, fishy, ammoniacal odor at low concentration.It's a colourless liquid with a boiling point around 3.5°C, compared with the higher melting point of 224-226°C for the more polar Me3NO, which presumably has dipole-dipole intermolecular forces.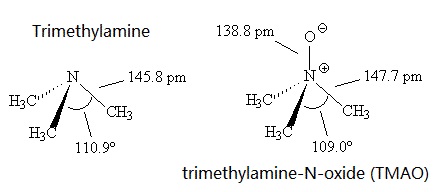
Trimethylamine is a base, like ammonia. Also like ammonia, it has a trigonal pyramidal structure. The C-N-C bond angle is 110.9°, compared with 107.2° in NH3, presumably due to greater repulsions between the methyl groups. This angle is reduced to 109.0° in Me3NO.
출처
TMA is widely distributed in the environment as a normal constituent of animal and plant tissue and as a result of its formation during the decay of organic matter in plants, animals, fish, sewage and animal waste (Graedel 1978; Hippe et al 1977; Oremland et al 1982). The amine is formed primarily as the result of microbial degradation of the plant and animal constituents betaine and choline and from bacterial reduction of trimethylamine oxide, a common constituent of aquatic organisms. It also occurs naturally in a variety of foodstuffs and in tobacco smoke and these are the most likely sources of human exposure (HSDB 1988).Numerous strains of bacteria isolated from various sources have been found capable of growing on TMA (HSDB 1988). Degradation products formed under anaerobic conditions include dimethylamine, formaldehyde, formate and C02, while under aerobic conditions, TMA is converted to dimethylamine, ammonia and methane.
용도
Trimethylamine is used as a warning agent for natural gas, a synthetic flavor (fish) ingredient, and in the synthesis of photochemicals, choline salts, flotation agents, dyes, pesticides, ion-exchange resins, cationic starches, and intense sweeteners (HSDB 2006).Organic synthesis, especially of choline salts, warning agent for natural gas, manufacture of disinfectants, flotation agent, insect attractant, quaternary ammonium compounds, plastics.
정의
ChEBI: Trimethylamine is a tertiary amine that is ammonia in which each hydrogen atom is substituted by an methyl group. It has a role as a human xenobiotic metabolite and an Escherichia coli metabolite. It is a tertiary amine and a member of methylamines. It is a conjugate base of a trimethylammonium.생산 방법
Trimethylamine (TMA) is produced by several methods: from the reaction of ammonia and methanol; from paraformaldehyde and ammonium chloride; by the action of formaldehyde and formic acid on ammonia; and by the interaction of methanol and ammonia over a catalyst at high temperature (Hawley 1981; HSDB 1988). TMA is sold as an aqueous solution or as a liquefied gas (Windholz et al 1983) in which the aqueous solution is available as 25, 30, and 40% and anhydrous as 99% minimum. The impurities consist of ammonia at no more than 0.2% by weight of solution and formaldehyde at no more than 0.3% by wt. of solution (Rick 1985). U.S. production was estimated to be approximately 15,322 tons in 1984 (HSDB 1988).제조 방법
Trimethylamine can be synthesized from paraformaldehyde and ammonium chloride, by the reaction of formic acid, formaldehyde, and ammonia, and by interaction of methanol and ammonia with a catalyst at high temperature.화학 반응
Trimethylamine (TMA) has been used in the preparation of poly[9,9′-bis(6′-N,N,N-trimethylammonium)hexyl)fluorene-co-alt-4,7-(2,1,3-benzothiadiazole) dibromide] (PFBT), a water-soluble, cationic conjugated polymer used in label-free DNA microarrays. It can also be used to prepare benzyltrimethylammonium chloride, which then reacts with sodium ethoxide to form benzyltrimethylammonium ethoxide.The adsorption of TMA on the gold surface of trimethylsilylated barium nitrate-gold/titanosilicate catalyst acts as a promoter for the propylene epoxidation with oxygen and hydrogen.일반 설명
A colorless gas with a fishlike odor at low concentrations changing to ammonia-like odor at higher concentrations. Shipped as a liquid under its own vapor pressure. Contact with the unconfined liquid can cause frostbite from evaporative cooling or chemical type burns. The gasis corrosive and dissolves in water to form flammable, corrosive solutions. Gas is an asphyxiate by the displacement of air. Produces toxic oxides of nitrogen during combustion. Prolonged exposure to heat can cause the containers to rupture violently and rocket. Long-term inhalation of low concentrations or short -term inhalation of high concentrations has adverse health effects.공기와 물의 반응
Highly flammable and easily ignited. Water soluble.반응 프로필
TRIMETHYLAMINE neutralizes acids in exothermic reactions to form salts plus water. May be incompatible with isocyanates, halogenated organics, peroxides, phenols (acidic), epoxides, anhydrides, and acid halides. Flammable gaseous hydrogen may be generated in combination with strong reducing agents, such as hydrides. Contamination of an ethylene oxide tank with trimethylamine caused an explosion [BCISC Quart. Safety Summ., 1966, 37, 44].건강위험
TMA is formed naturally from the biodegradation of plants, fish and animal products, and is ingested in foods such as fish, or from foods containing TMA precursors [e.g. trimethylamine oxide (TMAO), choline, and L-carnitine], which are metabolized to TMA by enterobacteria (Bain et al. 2005). Human exposure to TMA vapor has caused respiratory and eye irritation and corneal lesions. Effects in laboratory animals consisted of respiratory tract toxicity (gasping, labored breathing, lung lesions), eye lesions, neurotoxicity (apathy, splayed hind- or forelimbs, uncoordinated movements, convulsions, brain lesions), and some studies also found pathological changes of the liver, spleen, and kidneys.Safety Profile
Poison by intravenous route. Moderately toxic by subcutaneous and rectal routes. Mildly toxic by inhalation. A very dangerous fire hazard when exposed to heat or flame. Self-reactive. Moderately explosive in the form of vapor when exposed to heat or flame. Can react with oxidizing materials. To fight fire, stop flow of gas. Potentially explosive reaction with bromine + heat, ethylene oxide, triethynylaluminum. When heated to decomposition it emits toxic fumes of NOx. See also AMINES.잠재적 노출
Trimethylamine is used as a chemical intermediate in organic synthesis of quaternary ammonium com pounds; as an insect attractant; as a warning agent in natural gas; flotation agent.Carcinogenicity
No studies were found that examined the carcinogenicity of Trimethylamine in humans. Because mechanisms have been proposed by which the known carcinogen N47 nitrosodimethylamine can be formed from Trimethylamine and TMAO (Bain 2005) in the presence of nitrosating agents, there is some concern about the neoplastic potential of Trimethylamine. Thus, the German exposure guidelines warn that co-exposure to Trimethylamine and nitrosating agents should be minimized (see Section 8.2.). However, a 2-year mouse and rat inhalation study with the related amine DMA, which can also potentially form N-nitrosodimethylamine, showed no tumor formation despite severe chronic nasal lesions (CIIT 1990).Purification Methods
Dry triethylamine by passing the gas through a tower filled with solid KOH. Water and impurities containing labile hydrogen were removed by treatment with freshly sublimed, ground, P2O5. It has been refluxed with acetic anhydride, and then distilled through a tube packed with HgO and BaO. [Comyns J Chem Soc 1557 1955.] For more extensive purification, trimethylamine is converted to the hydrochloride, crystallised (see below), and regenerated by treating the hydrochloride with excess aqueous 50% KOH, the gas is passed through a CaSO4 column into a steel cylinder containing sodium ribbon. After 1-2 days, the cylinder is cooled to -78o and hydrogen and air are removed by pumping. [Day & Felsing J Am Chem Soc 72 1698 1950.] Me3N has been distlled from trap-to-trap and degassed by freeze-pump-thaw [Halpern et al. J Am Chem Soc 108 3907 1986]. It is commercially supplied in a pressure tin. [Beilstein 4 H 43, 4 I 322, 4 II 553, 4 III 99, 4 IV 134.]비 호환성
A medium strong base. Violent reaction with strong oxidizers (such as chlorine, bromine, fluorine), ethylene oxide; nitrosating agents, for example, nitrites, sodium nitrite, nitrous gases, nitrous acid) capable of releasing carcinogenic nitrosamines.); keep away from mercury, strong acids. Corrosive to many metals, for example, zinc, brass, aluminum, copper, tin, and their alloys.폐기물 처리
Return refillable compressed gas cylinders to supplier. Nonrefillable cylinders should be disposed of in accordance with local, state and federal regulations. Allow remaining gas to vent slowly into atmosphere in an unconfined area or exhaust hood. Refillabletype cylinders should be returned to original supplier with any valve caps and outlet plugs secured and valve protection caps in place.트라이메틸아민(트리메틸아민) 준비 용품 및 원자재
원자재
준비 용품
trimethyl p-dodecyl benzyl ammonium chloride
베타인
polysulfone anion exchange membrane
아세틸콜린브로마이드;에탄아미늄,2-(아세틸옥시)-N,N,N-트라이메틸-,브로마이드(1:1);(2-아세톡시에틸)트라이메틸암모늄브로마이드
Carbachol
METHACHOLINE CHLORIDE
2-하이드록시-N,N,N-트리메틸에탄아미늄 수산화물
알라클로르
Anion exchange resin,strong basic styrene
메틸 파라싸이온(메틸 파라티온)
술포텝
아세틸콜린아이오다이드
파라싸이온(파라티온)
N,N-DiMethylMethyleneaMMoniuM Iodide
염화트리메틸벤질암모늄
Betaine hydrochloride
헥사데실트리메틸암모늄 브롬
Triazophos E.C.
트리메틸아민옥사이드
염화콜린
(±)-카르니틴 하이드로클로라이드
Miltefosine
N,N'-헥사메틸렌비스(트라이메틸암모늄)
benzyltrimeehyl ammonium chloride
trimethyl α-hexadecyl betaine
styrene type polyethylene homogeneous anion exchange membrane
수산화 세트리모늄
DOWEX(R) 1X8
cationic fatliquor agent DLF-4
chloroalcohol type homogeneous strongly basic anion exchange membrane
DL-캐리틴
클로메쿼드
(N-헥실)트리메틸암모늄브로마이드
소듐사이클라메이트
트리메틸아민 하이드로클로라이드
interpenetrating network ion exchange resin
염화아세틸콜린
트리아조포스
(카르복실메틸)트리메틸암모늄 클로라이드 하이드라지드
2,5-Dichloropyridine
트라이메틸아민(트리메틸아민) 공급 업체
글로벌( 264)공급 업체
| 공급자 | 전화 | 이메일 | 국가 | 제품 수 | 이점 |
|---|---|---|---|---|---|
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 |
info@tianfuchem.com | China | 21688 | 55 |
| Shanghai Time Chemicals CO., Ltd. | +86-021-57951555 +8617317452075 |
jack.li@time-chemicals.com | China | 1807 | 55 |
| career henan chemical co | +86-0371-86658258 |
sales@coreychem.com | China | 29914 | 58 |
| Hubei Jusheng Technology Co.,Ltd. | 18871490254 |
linda@hubeijusheng.com | CHINA | 28180 | 58 |
| Hebei Guanlang Biotechnology Co., Ltd. | +86-19930503282 |
alice@crovellbio.com | China | 8829 | 58 |
| Hubei xin bonus chemical co. LTD | 86-13657291602 |
linda@hubeijusheng.com | CHINA | 22968 | 58 |
| Chongqing Chemdad Co., Ltd | +86-023-61398051 +8613650506873 |
sales@chemdad.com | China | 39916 | 58 |
| CONIER CHEM AND PHARMA LIMITED | +8618523575427 |
sales@conier.com | China | 49391 | 58 |
| Antai Fine Chemical Technology Co.,Limited | 18503026267 |
info@antaichem.com | CHINA | 9641 | 58 |
| Hebei Mojin Biotechnology Co., Ltd | +8613288715578 |
sales@hbmojin.com | China | 12468 | 58 |