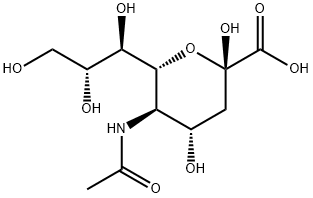
아세틸뉴라미닉애씨드
|
|
아세틸뉴라미닉애씨드 속성
- 녹는점
- 184-186 °C
- 끓는 점
- 449.56°C (rough estimate)
- 알파
- -32 º (c=2,water)
- 밀도
- 1.3580 (rough estimate)
- 굴절률
- -32 ° (C=1, H2O)
- 저장 조건
- -20°C
- 용해도
- 50g/L(20°C)
- 물리적 상태
- 합성, 결정질
- 산도 계수 (pKa)
- 2.41±0.54(Predicted)
- 색상
- 흰색에서 황백색까지
- 수용성
- 50g/L(20℃)
- 감도
- Air Sensitive
- Merck
- 14,8484
- BRN
- 1716283
- 안정성
- 안정적인. 강한 산화제와 호환되지 않습니다.
- InChIKey
- SQVRNKJHWKZAKO-PFQGKNLYSA-N
- LogP
- -1.897 (est)
- CAS 데이터베이스
- 131-48-6(CAS DataBase Reference)
안전
- 위험 및 안전 성명
- 위험 및 사전주의 사항 (GHS)
| 위험품 표기 | Xi | ||
|---|---|---|---|
| 위험 카페고리 넘버 | 36/37/38 | ||
| 안전지침서 | 22-24/25-36-26 | ||
| WGK 독일 | 3 | ||
| F 고인화성물질 | 3-10-23 | ||
| 위험 참고 사항 | Irritant | ||
| TSCA | Yes | ||
| HS 번호 | 29329970 |
아세틸뉴라미닉애씨드 C화학적 특성, 용도, 생산
개요
N-acetylneuraminic acid is an N-acyl derivative of neuraminic or acid amino sugar derivative, derived from N-acetylmannosamine and pyruvic acid. It is an important constituent of glycoproteins and glycolipids. N-acetylneuraminic acid occurs in many polysaccharides, glycoproteins, and glycolipids in animals and bacteria.물리적 성질
The numbering of the sialic acid structure begins at the carboxylate carbon and continues around the chain. The configuration which places the carboxylate in the axial position is the alpha-anomer.The alpha-anomer is the form that is found when sialic acid is bound to glycans, however, in solution it is mainly (over 90 %) in the beta-anomeric form. A bacterial enzyme with sialic acid mutarotase activity, NanM, has been discovered which is able to rapidly equilibrate solutions of sialic acid to the resting equilibrium position of around 90 % beta 10 % alpha.
Biosynthesis
In bacterial systems, sialic acids are biosynthesized by an aldolase enzyme. The enzyme uses a mannose derivative as a substrate, inserting three carbons from pyruvate into the resulting sialic acid structure. These enzymes can be used for chemoenzymatic synthesis of sialic acid derivatives.Biological Functions
Sialic acid-rich glycoproteins (sialoglycoproteins) bind selectin in humans and other organisms. Metastatic cancer cells often express a high density of sialic acid-rich glycoproteins. This overexpression of sialic acid on surfaces creates a negative charge on cell membranes. This creates repulsion between cells (cell opposition) and helps these late-stage cancer cells enter the blood stream.Sialic acid also plays an important role in human influenza infections.
Many bacteria also use sialic acid in their biology, although this is usually limited to bacteria that live in association with higher animals (deuterostomes). Many of these incorporate sialic acid into cell surface features like their lipopolysaccharide and capsule, which helps them evade the innate immune response of the host.[6] Other bacteria simply use sialic acid as a good nutrient source, as it contains both carbon and nitrogen and can be converted to fructose-6- phosphate, which can then enter central metabolism.
Sialic acid-rich oligo saccharides on the glyco conjugates ( glyco lipids, glyco proteins, proteoglycans) found on surface membranes help keep water at the surface of cells . The sialic acid - rich regions contribute to creating a negative charge on the cells' surfaces. Since water is a polar molecule with partial positive charges on both hydrogen atoms, it is attracted to cell surfaces and membranes. This also contributes to cellular fluid uptake.
Sialic acid can "hide" mannose antigens on the surface of host cells or bacteria from mannose - binding lectin . This prevents activation of complement.
Sialic acid in the form of poly sialic acid is an unusual posttranslational modification that occurs on the neural cell adhesion molecules (NCAMs). In the synapse, the strong negative charge of the polysialic acid prevents NCAM cross-linking of cells.
아세틸뉴라미닉애씨드 준비 용품 및 원자재
원자재
준비 용품
아세틸뉴라미닉애씨드 공급 업체
글로벌( 781)공급 업체
| 공급자 | 전화 | 이메일 | 국가 | 제품 수 | 이점 |
|---|---|---|---|---|---|
| BINBO BIOLOGICAL CO.,LTD | +8618629063126 |
info@binbobiological.com | China | 346 | 58 |
| Hebei Kingfiner Technology Development Co.Ltd | +86-15532196582 +86-15373005021 |
lisa@kingfinertech.com | China | 3001 | 58 |
| Hebei Mojin Biotechnology Co., Ltd | +8613288715578 |
sales@hbmojin.com | China | 12471 | 58 |
| Firsky International Trade (Wuhan) Co., Ltd | +8615387054039 |
admin@firsky-cn.com | China | 436 | 58 |
| Henan Bao Enluo International TradeCo.,LTD | +86-17331933971 +86-17331933971 |
deasea125996@gmail.com | China | 2503 | 58 |
| Shaanxi Haibo Biotechnology Co., Ltd | +undefined18602966907 |
qinhe02@xaltbio.com | China | 1000 | 58 |
| Hebei Jingbo New Material Technology Co., Ltd | +8619931165850 |
hbjbtech@163.com | China | 1000 | 58 |
| Hangzhou Zelixir Biotech Co., Ltd. | +8618867646786 |
neal.chen@zelixir.com | China | 230 | 58 |
| Chongqing Zhihe Biopharmaceutical Co., Ltd. | +86-18580541567 +86-17782035140 |
sales@zhswyy.com | China | 326 | 58 |
| Henan Fengda Chemical Co., Ltd | +86-371-86557731 +86-13613820652 |
info@fdachem.com | China | 18225 | 58 |