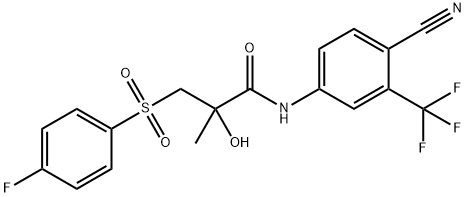
바이칼루타미드
|
|
바이칼루타미드 속성
- 녹는점
- 191-193°C
- 끓는 점
- 650.3±55.0 °C(Predicted)
- 밀도
- 1.52±0.1 g/cm3(Predicted)
- 저장 조건
- 2-8°C
- 용해도
- 에 용해됨 DMSO: >5mg/mL
- 산도 계수 (pKa)
- 11.49±0.29(Predicted)
- 물리적 상태
- 가루
- 색상
- 흰색에서 황백색까지
- 최대 파장(λmax)
- 270nm(CH3CN)(lit.)
- Merck
- 14,1200
- CAS 데이터베이스
- 90357-06-5(CAS DataBase Reference)
안전
- 위험 및 안전 성명
- 위험 및 사전주의 사항 (GHS)
| 위험품 표기 | Xi | ||
|---|---|---|---|
| 위험 카페고리 넘버 | 36/37/38 | ||
| 안전지침서 | 26-36-24/25 | ||
| 유엔번호(UN No.) | 3077 | ||
| WGK 독일 | 3 | ||
| RTECS 번호 | TX1413500 | ||
| 위험 등급 | 9 | ||
| 포장분류 | III | ||
| HS 번호 | 29242995 | ||
| 유해 물질 데이터 | 90357-06-5(Hazardous Substances Data) |
바이칼루타미드 C화학적 특성, 용도, 생산
개요
Bicalutamide was launched in the United Kingdom, its first worldwide market, for the treatment of advanced prostate cancer in combination with an LHRH analog or surgical castration. A non-steroidal, peripherally selective antiandrogen, bicalutamide inhibits the action of dihydrotestosterone and testosterone at target sites by competitive binding to the cytosolic androgen receptor. It was reportedly well tolerated with no significant cardiovascular and metabolic side effects due to the benefit of lacking any steroid activity. The efficacy of bicalutamide as a monotherapy has been demonstrated clinically. Promising response rates were also reported in treating colorectal, breast, pancreas and non-small cell lung cancers.화학적 성질
Off-White Crystalline Solid역사
Bicalutamide was discovered in the 1980s by Tucker et al. at Imperial Chemical Industries (now AstraZeneca). Based on previous works on flutamide, key structural features required for a strong anti-androgenic activity include the presence of an electron-poor aromatic ring, attached to an amide moiety. Electron-withdrawing groups at the para and the meta position of the anilide ring are beneficial for the anti-androgenic activity as compared to monosubstituted derivatives.As far as the meta position is concerned, a chloro or trifluoromethyl substituent is the best choice. Nitro and cyano groups are the best substituents at the para position. Replacement of themethyl group at the tertiary carbinol center by a trifluoromethyl group resulted in compounds with agonistic activity. In contrast to flutamide, the amide moiety of bicalutamide was extended by a sulfur linker with a second aromatic portion. The sulfanyl, sulfinyl, and sulfonyl analogues showed the same activity.The sulfanyl group was found to be oxidized to the active metabolite sulfonyl, thus indicating the sulfonyl derivative as the biologically active entity. An unsubstituted phenylsulfonyl moiety at the eastern part, or corresponding derivatives with small substituents such as fluoro at the para position, seemed to be the best in terms of anti-androgenic activity.용도
Bicalutamide (CDX) has been used as an androgen receptor (AR) antagonist in prostate, bladder cancer cell lines and human fetal skeletal muscle cells. It has also been used as a supplement in RPMI 1640 for culturing androgen-independent LNCaP (LNCaP-AI) cell line.Indications
Bicalutamide was the third nonsteroidal anti-androgen that was used for the treatment of prostate cancer. Flutamide, although effective in the treatment of prostate cancer, is a pure antagonist that also affects the hypothalamus pituitary axis, thus preventing the negative feedback mechanism of androgen. Consequently, the production of LH is increased, which subsequently stimulates the synthesis of testosterone, counteracting the effectiveness of the anti-androgen. Furthermore, the half-life of the active metabolite of flutamide, hydroxyflutamide, is fairly short, and a dosing scheme of 250 mg three times daily is therefore required. The main adverse effects reported for flutamide are gynecomastia, diarrhea, and reversible liver abnormalities. Nilutamide has a longer half-life than flutamide and therefore can be administered once daily. Adverse events reported include problems with light/dark adaptation and interstitial pneumonitis. The goal that ultimately led to the discovery of bicalutamide was the identification of a novel peripherally selective anti-androgen with longer half-life than flutamide and with better tolerability as compared to both, flutamide and nilutamide.정의
ChEBI: Bicalutamide is a sulfone that is an oral non-steroidal antiandrogen used in the treatment of prostate cancer and hirsutism.일반 설명
Bicalutamide, N-4-cyano-3-(trifluoromethyl)phenyl-3-[(4-fluorophenyl)sulfonyl]-2-hydroxy-2-methyl-propanamide (Casodex), is more potent than flutamideand has a much longer half-life (5.9 days vs. 6 hoursfor hydroxyflutamide). Because of the longer half-life, bicalutamideis used for once-a-day (50 mg) treatment of advancedprostate cancer. Bicalutamide is available as aracemic mixture, but both animal and human studies withthe AR show that the R-enantiomer has higher affinity forthe AR than the S-enantiomer.생물학적 활성
Orally active non-steroidal androgen receptor antagonist (IC 50 = 190 nM). Displays peripheral selectivity and does not effect serum levels of LH and testosterone. Exhibits potent anticancer activity in vivo .Mechanism of action
Bicalutamide is a racemate and its antiandrogenic activity resides almost exclusively in the (R)-enantiomer, which has an approximately fourfold higher affinity for the prostate AR than hydroxyflutamide does. The (S)-enantiomer has no antiandrogenic activity. (R)-Bicalutamide is slowly absorbed, but absorption is unaffected by food. It has a long plasma elimination half-life of 1 week and accumulates approximately 10 times in plasma during daily administration. (S)-Bicalutamide is much more rapidly absorbed and cleared from plasma. At steady state, the plasma levels of (R)-bicalutamide are 100 times higher than those of (S)-bicalutamide. Although mild to moderate hepatic impairment does not affect pharmacokinetics, evidence suggests slower elimination of (R)-bicalutamide in subjects with severe hepatic impairment.Pharmacology
Bicalutamide is a competitive AR antagonist, which shows in vitro a lower affinity for the AR as compared to the synthetic androgen R1881 as well as the natural DHT. However it displays a fourfold higher affinity as compared to hydroxyflutamide as assessed by a binding assay. Bicalutamide inhibits the growth of the LNCaP/FGC prostate carcinoma cell line, in which hydroxyflutamide was not effective at all. In vivo anti-androgenic activity of bicalutamide was confirmed by dose-dependent weight reduction of the seminal vesicles and ventrical prostate gland in rats, followed by an antitumor efficacy using Dunning R3327-GH prostate carcinomas in intact and castrated rats.A full overview on all clinical trials including bicalutamide would be out of scope.Clinical Use
Bicalutamide is a nonsteroidal pure antiandrogen given at a dosage of 150 mg once daily as monotherapy for the treatment of early (localized or locally advanced) nonmetastatic prostate cancer. It also can be used at a lower dosage in combination with a LHRH analogue or surgical castration for the treatment of advanced prostate cancer.부작용
Bicalutamide was well tolerated in monotherapy as well as in combination. No dose-related increase in adverse events was reported. Adverse events were partially due to pharmacological effects of an anti-androgen, which include gynecomastia, breast tenderness, and hot flushes. Other non-pharmacological adverse events, with incidence equal or higher than 10% were, for example, constipation, nausea, diarrhea, asthenia, pain, and infection. The frequency of non-pharmacological adverse events was in the same range as reported for comparator in clinical trials. In contrast to flutamide, the incidence of diarrhea and liver abnormalities was much lower for bicalutamide. As compared with castration, monotherapy with bicalutamide allowed patients to maintain libido and have better physical capacity, thus resulting in better quality of life.Based on the results of the clinical trials mentioned above, bicalutamide was first approved in 1995. Bicalutamide is indicated for the use in combination with an LHRH-A analogue for metastatic prostate carcinoma (50mg).신진 대사
Bicalutamide metabolites are excreted almost equally in urine and feces, with little or no unchanged drug excreted in urine. Unmetabolized drug predominates in the plasma. Following oral administration, the racemate displays stereoselective oxidative metabolism of its (R)-enantiomer, with an elimination half-life of approximately 6 days. (R)-Bicalutamide is cleared almost exclusively by CYP3A4-mediated metabolism, but glucuronidation is the predominant metabolic route for (S)-bicalutamide.바이칼루타미드 준비 용품 및 원자재
원자재
4-Fluorothiophenol
Expoxide
4-아미노벤조니트릴
메틸메타크릴레이트
4- 아미노 -2- (트리 플루오로 메틸) 벤조 니트릴
염화 티오닐
(R)-(-)-CITRAMALIC ACID
Tribromoacetaldehyde
준비 용품
바이칼루타미드 공급 업체
글로벌( 569)공급 업체
| 공급자 | 전화 | 이메일 | 국가 | 제품 수 | 이점 |
|---|---|---|---|---|---|
| Hebei Lingding Biotechnology Co., Ltd. | +86-18031140164 +86-19933155420 |
erin@hbldbiotech.com | China | 622 | 58 |
| Anhui Yiao New Material Technology Co., Ltd | +86-199-55145978 +8619955145978 |
sales8@anhuiyiao.com | China | 253 | 58 |
| Hebei Xinsheng New Material Technology Co., LTD. | +86-16632316109 |
xinshengkeji@xsmaterial.com | China | 1099 | 58 |
| Anhui Ruihan Technology Co., Ltd | +8617756083858 |
daisy@anhuiruihan.com | China | 994 | 58 |
| Nantong Guangyuan Chemicl Co,Ltd | +undefined17712220823 |
admin@guyunchem.com | China | 616 | 58 |
| Capot Chemical Co.,Ltd. | 571-85586718 +8613336195806 |
sales@capotchem.com | China | 29797 | 60 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 |
info@tianfuchem.com | China | 21688 | 55 |
| Hangzhou FandaChem Co.,Ltd. | 008657128800458; +8615858145714 |
fandachem@gmail.com | China | 9341 | 55 |
| Shanghai Yingrui Biopharma Co., Ltd. | +86-21-33585366 - 03@ |
sales03@shyrchem.com | CHINA | 738 | 60 |
| career henan chemical co | +86-0371-86658258 |
sales@coreychem.com | China | 29914 | 58 |
바이칼루타미드 관련 검색:
에틸 시아노아크릴산 페닐히드라진 2,3,5,6-테트라플루오로벤질 트랜스-2-(2,2-디클로로비닐)-3,3-디메틸사이클로프로판카르복실레이트 플루오르벤젠알데히드 메틸렌 티오사이아네이트 나트륨 사이아노브로민하이드라이드(나트륨 시아노브롬하이드리드) 프로피온아미드 트리플로르메틸-2벤조니트릴
CHLOROPHOSPHONAZO III
PHENYL VALERATE
Florfenicol
Bicalutamide intermediate F
N-[4-Cyano-3-(trifluoromethyl)phenyl]-3-[(4-fluorophenyl)thio]-2-hydroxy-2-methylpropionamide
N-[4-Cyano-3-(trifluoromethyl)phenyl]methacrylamide epoxide
BICALUTAMIDE-D4
Desfluoro Bicalutamide
Bicalutamide EP Impurity K
N-[4-Cyano-3-(trifluoromethyl)phenyl]-2-methacrylamide