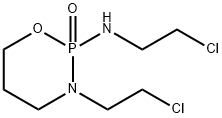
Ifosfamide
|
|
Ifosfamide 속성
- 녹는점
- 48°C
- 끓는 점
- 336.1±52.0 °C(Predicted)
- 밀도
- 1.33±0.1 g/cm3(Predicted)
- 저장 조건
- Inert atmosphere,2-8°C
- 용해도
- DMF:50.0(Max Conc. mg/mL);191.51(Max Conc. mM)
DMSO:44.0(Max Conc. mg/mL);168.53(Max Conc. mM)
Ethanol:51.0(Max Conc. mg/mL);195.34(Max Conc. mM)
PBS (pH 7.2):10.0(Max Conc. mg/mL);38.3(Max Conc. mM)
Water:52.0(Max Conc. mg/mL);199.17(Max Conc. mM)
- 산도 계수 (pKa)
- 1.44±0.20(Predicted)
- 물리적 상태
- Solid
- 색상
- White to off-white
- CAS 데이터베이스
- 3778-73-2(CAS DataBase Reference)
- IARC
- 3 (Vol. 26, Sup 7) 1987
안전
- 위험 및 안전 성명
- 위험 및 사전주의 사항 (GHS)
| 위험품 표기 | T | ||
|---|---|---|---|
| 위험 카페고리 넘버 | 25-36 | ||
| 안전지침서 | 26-45 | ||
| 유엔번호(UN No.) | 3249 | ||
| WGK 독일 | 3 | ||
| RTECS 번호 | RP6050000 | ||
| 위험 등급 | 6.1(b) | ||
| 포장분류 | III | ||
| HS 번호 | 29349990 | ||
| 유해 물질 데이터 | 3778-73-2(Hazardous Substances Data) | ||
| 독성 | LD50 in rats (mg/kg): 160 i.p. (Arnold, 1973); also reported as 150 i.p. (Brock) |
Ifosfamide C화학적 특성, 용도, 생산
화학적 성질
Crystalline Solid용도
A cytostatic agent, related structurally to cyclophosphamideIndications
Ifosfamide (Ifex) is an analogue of cyclophosphamide that requires metabolic activation to form 4-hydroxyifosfamide. In general, the metabolism, serum half-life, and excretion of ifosfamide are similar to those of cyclophosphamide.Ifosfamide is active against a broad spectrum of tumors, including germ cell cancers of the testis, lymphomas, sarcomas, and carcinomas of the lung, breast, and ovary. It is thought to be more active than cyclophosphamide in germ cell cancers and sarcomas.
Ifosfamide is less myelosuppressive than cyclophosphamide but is more toxic to the bladder. It also may produce alopecia, nausea, vomiting, infertility, and second tumors, particularly acute leukemias. Neurological symptoms including confusion, somnolence, and hallucinations have also been reported. It is recommended that ifosfamide be coadministered with the thiol compound mesna (Mesnex) to avoid hemorrhagic cystitis.
정의
ChEBI: The simplest member of the class of ifosfamides that is 1,3,2-oxazaphosphinan-2-amine 2-oxide substituted by 2-chloroethyl groups on both the nitrogen atoms respectively. It is a nitrogen mustard alkylating agent used in the treatment of advanced breast c ncer.일반 설명
Ifosfamide is available in 1- and 3-g vials for IV administrationas Food and Drug Administration (FDA)-approvedthird-line therapy in the treatment of testicular cancer. It has also been utilized (although not FDA approved) in the treatmentof a wide variety of cancers including Hodgkin’s andnon-Hodgkin’s lymphoma, soft tissue sarcoma, germ celltumors, small cell lung cancer, non–small cell lung cancer(NSCLC), cancers of the head and neck, bladder cancer, cervicalcancer, and Ewing sarcoma. Coadministration ofmesna is recommended. The mechanisms of resistance areidentical to those seen with cyclophosphamide. The drug iswidely distributed with a low extent of protein binding(20%). Metabolism primarily by CYP3A4/5 and CYP2B6 isrequired for activation of the compound. Theagent is administered as the racemic mixture as a result ofthe presence of the chiral phosphorus atom, and differentialmetabolism of the R- and S-isomers has been observed. Incontrast to cyclophosphamide, there is a greater amount ofdeactivation of the agent by N-dechloroethylation and subsequentlymore chloroacetaldehyde is produced, which mayresult in a greater amount of neurotoxicity and nephrotoxicitythan seen with cyclophosphamide. The N-dechloroethylatedmetabolites are the predominate species seen in theplasma. The parent and metabolites are eliminated inthe urine with an elimination half-life of 3 to 10 hours. Themajor components found in the urine are the dechlorethylatedmetabolites. Dose-limiting toxicities include myelosuppressionand bladder toxicity. Other adverse effectsinclude nausea, alopecia, amenorrhea, inappropriate secretionof antidiuretic hormone, as well as the production ofsecondary cancers. Neurotoxicity, which is associated withthe production of chloroacetaldehyde presents as confusion,seizure, weakness, and hallucination, and coma may occur.Clinical Use
Ifosamide currently is used as “third-line” therapy against testicular cancer, although it also has shown activity in a number of solid tumors and hematologic cancers.부작용
Patients on ifosfamide (but not cyclophosphamide) commonly exhibit central nervous system (CNS) toxicity. In severe forms, CNS depression can progress to coma and death.Ifosfamide 준비 용품 및 원자재
원자재
트리에틸아민
N-(2-Chloroethyl)amine HCl
1-Aziridinepropanol
3-(2-Chloroactyl)-2-[(2-chloroethyl)amino]tetrahydro-2H-1,3,2-oxazaphosphorine-2-oxide
2H-1,3,2-Oxazaphosphorine, 2-(1-aziridinyl)-3-(2-chloroethyl)tetrahydro-, 2-oxide
IFOSFAMIDE불순물F
데클로로에틸시클로포스파미드
준비 용품
Ifosfamide 공급 업체
글로벌( 485)공급 업체
| 공급자 | 전화 | 이메일 | 국가 | 제품 수 | 이점 |
|---|---|---|---|---|---|
| Hebei Chuanghai Biotechnology Co,.LTD | +86-13131129325 |
sales1@chuanghaibio.com | China | 5893 | 58 |
| Shanghai Daken Advanced Materials Co.,Ltd | +86-371-66670886 |
info@dakenam.com | China | 18648 | 58 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 |
info@tianfuchem.com | China | 21639 | 55 |
| career henan chemical co | +86-0371-86658258 +8613203830695 |
sales@coreychem.com | China | 29886 | 58 |
| SHANDONG ZHI SHANG CHEMICAL CO.LTD | +86 18953170293 |
sales@sdzschem.com | China | 2930 | 58 |
| Jinan Shengqi pharmaceutical Co,Ltd | 86+18663751872 |
christine@shengqipharm.com | CHINA | 491 | 58 |
| Xiamen AmoyChem Co., Ltd | +86-86-5926051114 +8618959220845 |
sales@amoychem.com | China | 6383 | 58 |
| Hubei xin bonus chemical co. LTD | 86-13657291602 |
linda@hubeijusheng.com | CHINA | 22963 | 58 |
| BOC Sciences | +1-631-485-4226 |
inquiry@bocsci.com | United States | 19553 | 58 |
| Beijing Yibai Biotechnology Co., Ltd | 0086-182-6772-3597 |
sales04@yibaibiotech.com | CHINA | 419 | 58 |
Ifosfamide 관련 검색:
글리신 베타인 산화 질소 시클로포스파미드 디에틸알루미늄클로라이드 시클로포스파미드, 모노수화물 클로로(2-)에틸벤젠
superoxide dismutase
ALTRENOGEST
Isocyclosporin A
ISOLIQUIRITIN
2-Chloroethanamine
Testosterone isocaproate
Sodium erythorbate
IFOSFAMIDE IMPURITY F
IFOSFAMIDE IMPURITY B
IFOSFAMIDE IMPURITY E
(1R,1'R)-2,2'-(3,11-Dioxo-4,10-dioxatridecamethylene)-bis-(1,2,3,4-tetrahydro-6,7-dimethoxy-1-veratrylisoquindline)-dioxalate