- TAK 599
-

- $7.00 / 1kg
-
2019-07-06
- CAS: 400827-46-5
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 100kg
|
| | TAK 599 Basic information |
| Product Name: | TAK 599 | | Synonyms: | TAK 599;Ceftaroline fosaMil;PPI 0903;TAK-599 (ceftaroline fosamil);Ceftaroline Fosamil Acetate;C22H22N8O8PS4. C2H3O2;TAK-599; CEFTAROLINE FOSAMIL;TAK599; TAK 599;PPI-0903;PPI0903;PPI 0903;Ceftaroline fosamil acetate salt | | CAS: | 400827-46-5 | | MF: | C24H25N8O10PS4 | | MW: | 744.72 | | EINECS: | | | Product Categories: | | | Mol File: | 400827-46-5.mol | 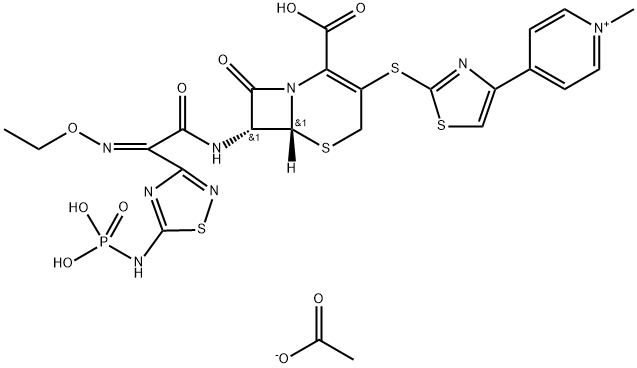 |
| | TAK 599 Chemical Properties |
| storage temp. | Store at -20°C | | solubility | DMSO : 30 mg/mL (40.28 mM; Need ultrasonic and warming) | | form | Powder |
| | TAK 599 Usage And Synthesis |
| Description | Ceftaroline fosamil, also referred to as TAK-599, is a cephalosporin
antibacterial agent that was approved in the United States in October
2010 for the IV treatment of acute bacterial skin and skin structure infections
(ABSSSI) and community-acquired bacterial pneumonia (CABP).
Ceftaroline fosamil is the water-soluble, N-phosphono prodrug of ceftaroline
(T-91825), a broad-spectrum, bactericidal agent with potent activity
against methicillin-resistant Staphylococcus aureus (MRSA) strains, multidrug
resistant S. pneumonia, and common gram-negative organisms.
Ceftaroline binds to PBP2a as well as other PBPs with high
affinity and, as a result, retains potent activity. Ceftaroline exhibits activity
against most gram-positive pathogens, including β-lactam-susceptible
and -resistant S. aureus, vancomycin-resistant S. aureus, and resistant and
susceptible forms of S. pneumoniae but has weak activity against Enterococcus
sp. The gram-negative antibacterial activity of ceftaroline is limited mainly
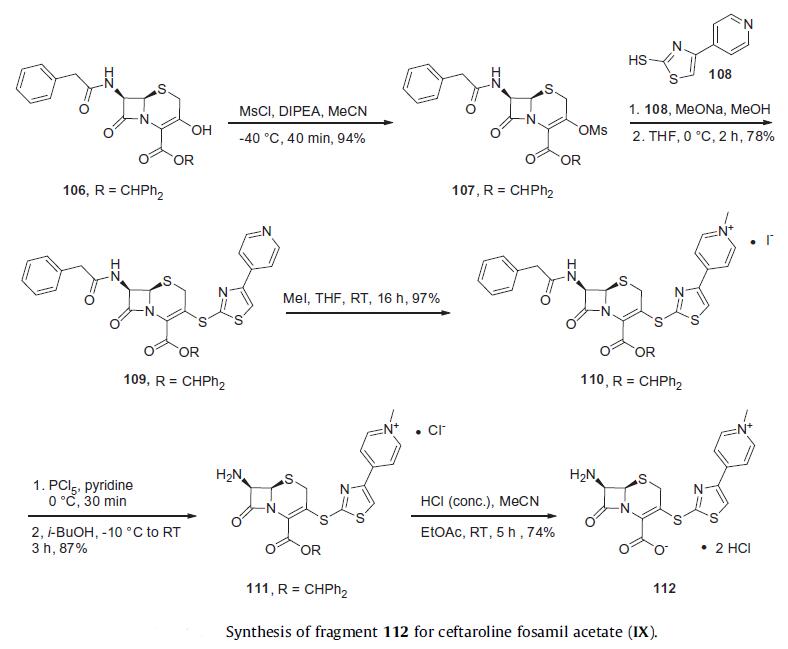
to respiratory pathogens such as Moraxella catarrhalis and Haemophilus influenzae. | | Originator | Takeda (Japan) | | Definition | ChEBI: An acetate salt obtained by reaction of ceftaroline fosamil with one equivalent of acetic acid. A prodrug for ceftaroline, used for the treatment of adults with acute bacterial skin and skin structure infections. | | Brand name | Teflaro | | Synthesis | Reports from Takeda describe a process preparation of ceftaroline
fosamil in 100 g scale which relies upon the assembly and union
of fragments 112 and 114. The
synthesis of fragment 112 began from commercially available
benzhydryl 7b-[(phenylacetyl)amino]-3-hydroxy-3-cephem-4-
carboxylate (106). The hydroxyl group within cephem 106 was reacted
with methanesulfonyl chloride to produce mesylate 107 in
94% yield. The condensation of mesylate 107 with 4-(pyridin-
4-yl)thiazole-2-thiol 108 under the base condition of sodium
methoxide gave compound 109 in 78% yield. Pyridinium salt 110 arose in quantitative yield upon subjection of 109 to iodomethane.
Sequential deprotections of the amino group with phosphorous
pentachloride and ester group with concentrated HCl afforded
the dihydrochloride salt 112 in good yield.

Acyl halide fragment 114 was prepared from commercially
available (Z)-2-(5-amino-1,2,4-thiadiazol-3-yl)-2-ethoxyiminoacetic
acid (113) in 60% yield by dichlorophosphorylation of the amino
group and concomitant acid chloride formation. Acid chloride 114
was then reacted with dihydrochloride salt 112 in the presence of
sodium acetate to give N-phosphono cephem 115 in 77% yield. The
crystallization of 115 in an aqueous acetic acid solution gave rise to
the stable acetic acid solvate ceftaroline fosamil acetate (IX). | | Drug interactions | Potentially hazardous interactions with other drugs
Anticoagulants: effects of coumarins may be
enhanced. | | Metabolism | Ceftaroline fosamil (prodrug) is converted into the active
ceftaroline in plasma by phosphatase enzymes. Hydrolysis
of the beta-lactam ring of ceftaroline occurs to form
the microbiologically inactive, open-ring metabolite,
ceftaroline M-1.
Ceftaroline is mainly eliminated by the kidneys. Renal
clearance is approximately equal, or slightly lower than
the glomerular filtration rate in the kidney, and in vitro
transporter studies indicate that active secretion does not
contribute to the renal elimination of ceftaroline. |
| | TAK 599 Preparation Products And Raw materials |
|