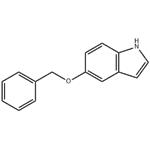
- 5-Benzyloxyindole
-
- $10.00 / 1KG
-
2024-04-28
- CAS:1215-59-4
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: g-kg-tons, free sample is available
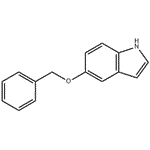
- 5-Benzyloxyindole
-
- $1.50 / 1g
-
2023-07-27
- CAS:1215-59-4
- Min. Order: 1g
- Purity: 99.0% Min
- Supply Ability: 100 Tons
- 5-Benzyloxyindole
-

- $24.00 / 1kg
-
2022-12-28
- CAS:1215-59-4
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 1000kg
|
| | 5-Benzyloxyindole Basic information |
| | 5-Benzyloxyindole Chemical Properties |
| Melting point | 100-104 °C (lit.) | | Boiling point | 364.56°C (rough estimate) | | density | 1.0707 (rough estimate) | | refractive index | 1.5500 (estimate) | | storage temp. | 2-8°C | | solubility | ethanol: may be hazy yellow | | form | powder | | pka | 16.63±0.30(Predicted) | | color | white to light yellow | | BRN | 173532 | | Exposure limits | ACGIH: TWA 20 ppm
OSHA: Ceiling 300 ppm; TWA 200 ppm
NIOSH: IDLH 500 ppm; TWA 100 ppm(375 mg/m3); STEL 150 ppm(560 mg/m3) | | CAS DataBase Reference | 1215-59-4(CAS DataBase Reference) | | NIST Chemistry Reference | 1H-indole, 5-(phenylmethoxy)-(1215-59-4) |
| Hazard Codes | Xi | | Risk Statements | 36/37/38 | | Safety Statements | 26-24/25-22 | | WGK Germany | 3 | | RTECS | NL4850000 | | F | 8-10 | | HazardClass | IRRITANT | | HS Code | 29339990 |
| | 5-Benzyloxyindole Usage And Synthesis |
| Chemical Properties | Off-white to beige powder | | Uses | 5-Benzyloxyindole is used as reagent/reactant in regioselective preparation of CF3-β-tryptamine derivatives via base-free thermal ring-opening reaction of N-nosyl-2-CF3-aziridine with indoles. | | Uses | - Reactant in regio- and stereoselective morpholine-catalyzed direct C-3 alkenylation with α,β-unsaturated aldehydes
- Reactant in selective debenzylation of protective groups using SiliaCat-palladium under mild reaction conditions
- Reactant in metal-free Friedel-Crafts alkylation reactions
- Reactant in preparation of protein kinase (PKC) inhibitors
- Reactant in preparation of indole/quinoline carbothioic acid amide derivatives
| | Uses | Reactant in regio- and stereoselective morpholine-catalyzed direct C-3 alkenylation with α,β-unsaturated aldehydes Reactant in selective debenzylation of protective groups using SiliaCat-palladium under mild reaction conditions Reactant in metal-free Friedel-Crafts alkylation reactions Reactant in preparation of protein kinase (PKC) inhibitors Reactant in preparation of indole/quinoline carbothioic acid amide derivatives. | | Purification Methods | It is recrystallised from *C6H6/pet ether or pet ether. The picrate forms red crystals from *C6H6 and has m 142-143o. [Burton & Leong Chem Ind (London) 1035 1953, Ek & Witkop J Am Chem Soc 76 5579 1954, fluorescence: Bridges & Williams Biochem J 107 225 1968, Beilstein 27 III/IV 1758.] |
| | 5-Benzyloxyindole Preparation Products And Raw materials |
|