- Nimodipine
-

- $48.00 / 100mg
-
2024-10-31
- CAS:66085-59-4
- Min. Order:
- Purity: 99.71%
- Supply Ability: 10g
- Nimodipine
-

- $48.00 / 100mg
-
2024-10-31
- CAS:66085-59-4
- Min. Order:
- Purity: 99.71%
- Supply Ability: 10g
- Nimodipine
-

- $999.00/ kg
-
2024-10-31
- CAS:66085-59-4
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 5000
|
| Product Name: | Nimodipine | | Synonyms: | 1,4-Dihydro-2,6-dimethyl-4-(3-nitrophenyl)pyridine-3,5-dicarboxylic acid 3-isopropyl 5-(2-methoxyethyl) ester;3,5-Pyridinedicarboxylic acid, 1,4-dihydro-2,6-dimethyl-4-(3-nitrophenyl)-, 3-(2-methoxyethyl) 5-(1-methylethyl) ester;Nimodipine,1,4-Dihydro-2,6-dimethyl-4-(3-nitrophenyl)-3,5-pyridinedicarboxylic acid 2-methoxyethyl 1-methylethyl ester, Isopropyl 2-methoxyethyl 1,4-dihydro-2,6-dimethyl-4-(m-nitrophenyl)-3,5-pyridine;Nimodipime;Isopropyl 2-methoxyethyl 2,6-dimethyl-4-(3-nitrophenyl)-1,4-dihydropyridine-3,5-dicarboxylate;Nimodipine (125 mg);NiModipine(NiMotop);1,4-Dihydro-2,6-diMethyl-4-(3-nitrophenyl)- | | CAS: | 66085-59-4 | | MF: | C21H26N2O7 | | MW: | 418.44 | | EINECS: | 266-127-0 | | Product Categories: | Cardiovascular APIs;Pharmaceutical intermediate;API;Ion Channels;Aromatics;Dihydropyridine;Isotope Labelled Compounds;Calcium channel;Dihydropyridine Class Chemicals;Inhibitors;Intermediates & Fine Chemicals;Pharmaceuticals;API's | | Mol File: | 66085-59-4.mol | 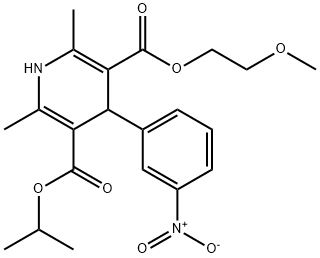 |
| | Nimodipine Chemical Properties |
| Melting point | 125°C | | Boiling point | 536.21°C (rough estimate) | | density | 1.3268 (rough estimate) | | refractive index | 1.6500 (estimate) | | storage temp. | 2-8°C | | solubility | methanol: 62.5 mg/mL | | form | solid | | pka | 2.77±0.70(Predicted) | | color | white | | Merck | 14,6551 | | Stability: | Stable for 2 years from date of purchase as supplied. Solutions in DMSO may be stored at -20°C for up to 3 months. | | InChIKey | UIAGMCDKSXEBJQ-UHFFFAOYSA-N | | CAS DataBase Reference | 66085-59-4(CAS DataBase Reference) | | NIST Chemistry Reference | Nimodipine(66085-59-4) |
| Hazard Codes | Xn | | Risk Statements | 20/21/22-48/22 | | Safety Statements | 36 | | WGK Germany | 1 | | RTECS | US7975500 | | HazardClass | IRRITANT | | HS Code | 29333990 | | Toxicity | LD50 in mice, rats (mg/kg): 3562, 6599 orally; 33, 16 i.v. (Schlüter) |
| | Nimodipine Usage And Synthesis |
| Calcium channel blockers | Nimodipine, molecular formula C21H26N2O7, was first developed by Germany Bayer pharmaceutical industry. It is the latest generation of 1,4-dihydropyridine calcium channel blocker that is calcium blockers. It can inhibit the influx of calcium into vascular smooth muscle cells. It has bronchodilation effects isolated or intracorporal cerebral artery or ischemia cerebral artery. It can significantly improve cerebral blood flow, and effectively prevent and treat cerebral ischemic damage, migraines, sudden deafness that caused by cerebral vascular spasm which is induced by subarachnoid hemorrhage. Nimodipine can also be used for mild and moderate hypertension. It can effectively regulate the body's calcium in order to maintain the normal physiological function. It has particularly prominent effects on cerebral vascular, and it can combine with specific receptors of central nervous system. Cerebral vessels can be selectively expanded at a suitable dose, and it can hardly affect peripheral vascular. It also has a good effect on high blood pressure when its dose is increased.
The above information is edited by the Chemicalbook of Ge Qian. | | Pharmacological action | Nimodipine is calcium channel blockers. It can relieve vasospasm by effectively preventing Ca2+ into the cell and inhibiting contraction of smooth muscle. It is highly lipophilic, and easily permeates the blood-brain barrier. It has a strong effect on cerebral artery. In addition, it also has positive effect on protecting memory and recovering intelligence. Nimodipine has selective effects on cerebrovascular smooth muscle. It can expand cerebral blood vessels, increase cerebral blood flow and significantly reduce ischemic brain damage caused by vasospasm. | | Pharmacokinetics | 1. When taken orally, nimodipine can be rapidly absorbed after oral administration, and the concentration will reach a peak within about 1 hour. T1/2 is 1 to 2 hours, and the elimination time is 8 to 9 hours. After taken orally four times a day, the blood has no obvious accumulation of nimodipine for seven days. When its concentration is between 10ng/L and 10μg/mL, more than 95% of drugs combine with plasma protein. Most of nimodipine will be excreted in the form of metabolites after oral, and about 1% of the ingredients exit the body unchanged in the urine. Due to the rapid metabolism of nimodipine in a first phase, the bioavailability after taken orally is 13%. The biological activity of nimodipine in patients with chronic liver damage increases. And the maximum concentration can be up to twice as high as normal people.
2. Intravenous injection: The dosage for intravenous injection is 0.03mg/kg. The half-life (t1/2) is (1.1±0.2) h. According to the study, 15 patients with subarachnoid space bleeding (grade 1-3) have been injected with this product for 48mg a day, lasting 14 days. The average plasma concentration is 36~ 72μg/L. The protein binding rate is 96% to 99%. The steady state distribution of apparent volume is 1.6~3.1L/kg. The concentration of plasma drug decline quickly, and the metabolites almost has no activity. It is mainly eliminated through bile. 80% can be excreted by the intestine with faeces and 20% by the kidneys with the urine. | | Synthesis route | The first step: using hydrogen chloride, concentrated sulfuric acid or acetic acid-piperidine as a catalyst, m-nitrobenzaldehyde (2) and 2-methoxy-ethyl acetoacetate (3) generate 2-(3-nitrobenzylidene) ethyl 2-methoxyethyl acetoacetate (4) by condensation.
The second step: concentrated hydrochloric acid and absolute ethanol are heated to the reflux reaction conditions under nitrogen. (4) and 3-amino butyric acid isopropyl ester (5) are heated to obtain crude product of (1). After cooling crystallization, pumping filtered, the filter cake is washed with cold ethanol. Then it is dried to get pale yellow crystals nimodipine (1).

Figure 1: Synthesis route of nimodipine
| | Indications | Nimodipine is a calcium antagonist of selective expansion of cerebral blood vessels. It can increase cerebral blood flow, improve cerebral blood circulation, prevent ischemic cerebral vasospasm, reduce calcium influx, protect ischemic brain cell function, prevent the occurrence and development of brain vascular disease and delay and prevent the occurrence of stroke. Nimodipine is mainly used for cerebral insufficiency, cerebral vasospasm, subarachnoid hemorrhage, stroke and migraine. It has a certain effect on sudden deafness. | | Attentions | 1.Nimodipine should be used for patients with cerebral edema and intracranial hypertension with caution. Nimodipine metabolites has toxicity. So patients with liver dysfunction should be used with caution. Nimodipine can cause a lowering of blood pressure. For patients with hypertensive subarachnoid hemorrhage or stroke patients, the dosage of antihypertensive drugs should be paid attention to be reduced or temporarily stopped, or the dosage of nimodipine should be reduced. That can produce intestinal pseudo-obstruction, manifested as abdominal distension, decreased bowel sounds. When the above symptoms appears, the dosage should be reduced and observation should be hold.
2.Nimodipine can be secreted by milk. Breast-feeding women should not use. Animal experiments suggest that this product has teratogenicity. | | Untoward reaction | There are about 11.2% of patients with subarachnoid hemorrhage that have adverse reactions. The most common symptoms are blood pressure dropping (the extent of decline is related to drug dose), hepatitis, skin irritation, gastrointestinal bleeding, thrombocytopenia, occasionally transient dizziness, headache, facial flushing, vomit, gastrointestinal discomfort and the like. In addition, after taken nimodipine orally, individual patients may appear alkaline phosphatase (ALP), lactate dehydrogenase (LDH), blood sugar elevating and platelet count increasing for individual human. | | Usage and dosage | The usage and dosage of this product is very different for different symptom and severity.
1. Nimodipine can be taken orally when symptoms are mild:
Ischemic cerebrovascular disease: 20~40mg, 3 times/d, continuous use for a month.
Migraine: 40mg, 3 times/d; 12 weeks for a course of treatment.
Hypertension: 40~80mg, 3 times/d.
Sudden deafness: 10~20mg, 3 times/d; 5 days as a course of treatment, usually for 3 to 4 courses.
Cerebral vasospasm caused by subarachnoid hemorrhage: 10~20mg, 3~4 times/d; 3 to 4 weeks for a course of treatment.
2. Severe patients with the above-mentioned diseases may be considered to use ntravenous injection: the beginning of 0.5mg/h, gradually increase after 2h to 1~2mg/h; 5~10d later change to oral. | | Chemical property | Light yellow crystalline powder, odorless and tasteless. Soluble in ethanol or acetone, insoluble in water. Melting point 124~128℃. Acute toxicity LD50 in mice and rats (mg/kg): 3562, 6599. Oral: 33. Intravenous injection: 16.
(+)-Configuration: [α] D20+7.9° (C=0.439, dioxane).
(-)-Configuration: [α] D20-7.93° (C=0.374, dioxane). | | Uses | 1. Calcium channel blockers. Nimodipine has anti-ischemic and anti-vasoconstriction effect. It is the drug to improve cerebral vasodilator and brain function. It can be used for treating ischemic cerebrovascular disease, mild to moderate hypertension, migraine, cerebral vasospasm, sudden deafness and the like.
2. Calcium channel blockers. Nimodipine is mainly used for the treatment of ischemic cerebrovascular disease and migraine.
3. A new calcium antagonist. Nimodipine is used for the treatment of cerebrovascular disorders, such as cerebral vasospasm, interim and early ischemic cerebrovascular migraine, and sudden deafness. | | Production method | Method 1: Nimodipine can be synthesized from nitrobenzaldehyde first by condensation with methoxyethyl acetoacetate catalyzed by hydrochloric acid or concentrated sulfuric acid, and then heated with 3-amino butyric acid isopropyl ester in ethanol by cyclization.
Method 2: Nitrobenzaldehyde and isopropyl acetoacetate also can be dissolved with stirring at room temperature firstly. Then add glacial acetic acid and piperidine and stir at 40-50℃ to solidify (about 6~7h). After processing for 4h at 40-50℃, 95% ethanol is added, and then heated under reflux until the solid dissolved. Cooled to 0~5℃, precipitated crystals can be filtered and dried to give 2-(3-nitrophenyl methylene) acetyl isopropyl acetate. The yield is 90.6%. Then it reacts with 3-amino butyric acid and 2-methoxy-ethyl ester to obtain the crude product. The crude product can be recrystallized from ethanol to get nimodipine. The yield is 84%, and the melting point is 125~126℃.
The second step can also be carried out as follows: 3.8g 2-(3-nitrophenyl methylene) acetyl isopropyl acetate, 8g 2-methoxy-ethyl acetoacetate and 6ml of concentrated ammonia are heated at reflux for 8h in 80ml of ethanol. The product is recrystallized with petroleum ether-ethyl acetate to obtain nimodipine. The yield is 49%, and the melting point is 125℃. | | Description | Nimodipine is a cerebral vasodilating calcium antagonist related to nifedipine. It
is indicated for the prophylaxis and treatment of neurological deficits due to
cerebral vasospasm after subarachnoid hemorrhage. | | Chemical Properties | Crystalline Solid | | Originator | Bayer (W. Germany) | | Uses | provitamin, antixerophthalamic | | Uses | Nimodipine has been used:
- as a L-type calcium channel (LTCC) inhibitor, to evaluate its neuroprotective activity in the vibrosections
- as a standard, in the enantioseparation of chiral drugs by high performance liquid chromatography (HPLC)
- in the pharmacological studies for the measurement of spine voltage escape
| | Uses | Used as a cerebral vasodilator. A dihydropyridine calcium channel blocker | | Uses | A dihydropyridine calcium channel blocker. It is used as a vasodilator (cerebral). | | Definition | ChEBI: A dihydropyridine that is 1,4-dihydropyridine which is substituted by methyl groups at positions 2 and 6, a (2-methoxyethoxy)carbonyl group at position 3, a m-nitrophenyl group at position 4, and an isopropoxycarbonyl group at position 5. An L
type calcium channel blocker, it acts particularly on cerebral circulation, and is used both orally and intravenously for the prevention and treatment of subarachnoid hemorrhage from ruptured intracranial aneurysm. | | Manufacturing Process | After 8 hours boiling of solution of 3.8 g of 3'-nitro-benzylideneacetoacetic
acid isopropylester, 8 grams of acetoacetic acid β-metoxyethyl ester and 6 ml
conc ammonia in 80 ml ethanol under reflux, 2,6-dimethyl-4-(3'-nitrophenyl)-
1,4-dihydropyridine 3-β-methoxyethyl ester 5-isopropyl ester of melting point
125°C (petroleum ether/ acetic ester) was obtained. Yield 49% of theory. | | Brand name | Nimotop
(Bayer). | | Therapeutic Function | Vasodilator | | General Description | Nimodipine, 1,4-dihydro-2,6-dimethyl-4-(3-nitrophenyl)- 3,5-pyridinedicarboxylic acid 2-methoxyethyl1-methylethyl ester (Nimotop), is another dihydropyridinecalcium channel blocker but differs in that it dilatesthe cerebral blood vessels more effectively than do the otherdihydropyridine derivatives. This drug is indicated for treatmentof subarachnoid hemorrhage-associated neurologicaldeficits. | | Biological Activity | L-type Ca 2+ channel blocker. | | Biochem/physiol Actions | Nimodipine enhances the survival of dopaminergic substantia nigra neurons. | | Clinical Use | Calcium-channel blocker:
Prevention and treatment of ischaemic neurological
deficits following subarachnoid haemorrhage | | Drug interactions | Potentially hazardous interactions with other drugs
Aminophylline: possibly increases aminophylline
concentration.
Anaesthetics: enhanced hypotensive effect.
Antibacterials: metabolism accelerated by rifampicin;
metabolism possibly inhibited by clarithromycin,
erythromycin and telithromycin.
Antidepressants: enhanced hypotensive effect with
MAOIs .
Antiepileptics: effect reduced by carbamazepine,
barbiturates, phenytoin and primidone.
Antifungals: metabolism possibly inhibited by
itraconazole and ketoconazole; negative inotropic
effect possibly increased with itraconazole.
Antihypertensives: enhanced hypotensive effect,
increased risk of first dose hypotensive effect of post synaptic alpha-blockers.
Antivirals: concentration possibly increased by
ritonavir; use telaprevir with caution.
Grapefruit juice: concentration increased - avoid.
Theophylline: possibly increased theophylline
concentration. | | Metabolism | Nimodipine is extensively metabolised in the liver via the
cytochrome P450 isoenzyme CYP3A4. It is eliminated
as metabolites, mainly by dehydrogenation of the
dihydropyridine ring and oxidative O-demethylation.
Oxidative ester cleavage, hydroxylation of the 2- and
6-methyl groups, and glucuronidation as a conjugation
reaction are other important metabolic steps. The three
primary metabolites occurring in plasma show no or only
therapeutically negligible residual activity.
The metabolites are excreted about 50% renally and 30%
in faeces via the bile. | | storage | Store at RT | | References | 1) Cohen and McCarthy (1987),?Nimodipine block of calcium channels in rat anterior pituitary cells; J. Physiol.,?387?195
2) Batuecas?et al.?(1998),?Effects of chronic nimodipine on working memory of old rats in relation to defects in synaptosomal calcium homeostasis; Eur. J. Pharmacol.,?350?141
3) LeVere?et al.?(1989),?Recovery of function after brain damage: facilitation by the calcium entry blocker nimodipine; Behav. Neurosci.,?103?561
4) Herzfeld?et al.?(2014),?Investigation of the neuroprotective impact of nimodipine on Neuro2a cells by means of a surgery-like stress model; Int. J. Mol. Sci.,?15?18453
5) Schampel?et al.?(2017),?Nimodipine fosters remyelination in a mouse model of multiple sclerosis and induces microglia-specific apoptosis; Proc. Natl. Acad. Sci. USA,?114?E3295
6) Allen?et al.?(1983),?Cerebral arterial spasm – a controlled trial of nimodipine in patients with subarachnoid hemorrhage; N. England J. Med.,?308?619 |
| | Nimodipine Preparation Products And Raw materials |
|