- Naphazoline
-

- $0.00 / 1kg
-
2022-06-02
- CAS:835-31-4
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 1000kg
- naphazoline
-

- $50.00 / 1KG
-
2021-08-31
- CAS:835-31-4
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: 500tons/month
- Naphazoline
-

- $1.00 / 1KG
-
2020-03-06
- CAS:835-31-4
- Min. Order: 1KG
- Purity: 97%-99.9%
- Supply Ability: 100kg
|
| | Naphazoline Basic information |
| Product Name: | Naphazoline | | Synonyms: | 4,5-DIHYDRO-2-(1-NAPHTHYLMETHYL)-1H-IMIDAZOLE HYDROCHLORIDE;2-(1-NAPHTHYLMETHYL)IMIDAZOLINIUM CHLORIDE;1H-Imidazole, 4,5-dihydro-2-(1-naphthalenylmethyl)-;NAFAZOLINE;2-(Naphthalen-1-ylmethyl)-4,5-dihydro-1H-imidazole;Naphazoline;2-(1-Naphtylmethyl)-2-imidazoline;2-(1-Naphtylmethyl)-4,5-dihydro-1H-imidazole | | CAS: | 835-31-4 | | MF: | C14H14N2 | | MW: | 210.27 | | EINECS: | 212-641-5 | | Product Categories: | API | | Mol File: | 835-31-4.mol |  |
| | Naphazoline Chemical Properties |
| Melting point | 254 °C | | Boiling point | 339.81°C (rough estimate) | | density | 1.1063 (rough estimate) | | refractive index | 1.6180 (estimate) | | storage temp. | 2-8°C | | pka | pKa 10.35 ± 0.02(H2O,t =25,Iundefined) (Uncertain) | | CAS DataBase Reference | 835-31-4(CAS DataBase Reference) | | NIST Chemistry Reference | Naphazoline(835-31-4) |
| Hazard Codes | T | | Risk Statements | 25 | | Safety Statements | 45 | | RIDADR | UN 2811 6.1/PG 2 | | WGK Germany | 3 | | RTECS | NJ4375000 |
| | Naphazoline Usage And Synthesis |
| Originator | Privine,Ciba,US,1942 | | Uses | Naphazoline is used in severe rhinitis associated with colds, allergic reactions, and severe
and chronic inflammatory conditions, in particular for inflammation of the antrum of
Highmore as well as for stopping nosebleeds. | | Uses | Adrenergic (vasoconstrictor). | | Definition | ChEBI: 2-(1-naphthalenylmethyl)-4,5-dihydro-1H-imidazole is a member of naphthalenes. | | Manufacturing Process | 2.7 parts of naphthyl-(1)-acetiminoethylether hydrochloride of the formula
(produced from naphthyl-(1)-acetonitrile and methanol) are dissolved in 12
parts of absolute alcohol. 1 part of ethylenediamine is then added and the whole is heated to gentle boiling while passing nitrogen through it and
simultaneously stirring until ammonia escapes no longer. The alcohol is then
distilled and the residue mixed with 40 parts of benzene and 1.8 parts of
caustic potash. Stirring is continued for some time whereby the imidazoline
base is dissolved in benzene. The benzene residue is recrystallized several
times from toluene. | | Brand name | Albalon (Allergan); Nafazair
(Bausch & Lomb); Nafazair (Pharmafair); Naphcon
(Alcon); Opcon (Bausch & Lomb); Vasocon (Novartis). | | Therapeutic Function | Nasal decongestant | | Synthesis | Naphazoline, 2-(1-naphthylmethyl)-2-imidazoline (11.1.36), is synthesized
from (1-naphthyl)acetonitrile, which upon reaction with ethanol transforms into imi�noester (11.1.35), and undergoes further heterocyclization into the desired imidozoline
derivative (11.1.36) upon reaction with ethylendiamine [40]. 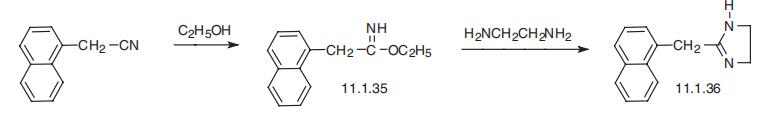
|
| | Naphazoline Preparation Products And Raw materials |
|