| Company Name: |
JinJin Le Chemical Co., Ltd
|
| Tel: |
10106090 |
| Email: |
jjlchem2@163.com |
| Products Intro: |
CAS:1311-11-1
Purity:On Request Package:电询
|
| Company Name: |
ChemService Inc.
|
| Tel: |
(800) 452-9994 Orders Only |
| Email: |
|
| Products Intro: |
Product Name:Lead hydroxide
CAS:1311-11-1
Remarks:Cat.:I-3520
|
|
| | LEAD HYDROXIDE Basic information |
| Product Name: | LEAD HYDROXIDE | | Synonyms: | LEAD HYDROXIDE;BASIC LEAD HYDROXIDE;plumbous hydroxide;lead(2+),dihydroxide | | CAS: | 1311-11-1 | | MF: | H2O2Pb | | MW: | 241.21 | | EINECS: | | | Product Categories: | | | Mol File: | 1311-11-1.mol | 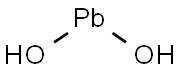 |
| | LEAD HYDROXIDE Chemical Properties |
| density | 7.41 | | solubility | insoluble in H2O; soluble in dilute acid solutions | | form | powder | | color | white | | Water Solubility | insoluble H2O; soluble dilute acid [CRC10] | | Solubility Product Constant (Ksp) | pKsp: 14.84 |
| | LEAD HYDROXIDE Usage And Synthesis |
| Chemical Properties | white powder(s) [CRC10] | | Physical properties | White amorphous powder; density 7.41 g/cm3; dehydrates above 130°C and decomposes at 145°C; slightly soluble in water, 155 mg/L at 20°C; KSP 1.42x10-20 at 25°C; soluble in dilute acids and alkalies; insoluble in acetone and acetic acid. | | Uses | Lead hydroxide is used in making porous glass; in electrical-insulating paper; in electrolytes in sealed nickel-cadmium batteries; in recovery of uranium from seawater; and as a catalyst for oxidation of cyclododecanol. | | Preparation | Lead dioxide is produced by oxidizing an alkaline slurry of lead monoxide with chlorine, sodium hypochlorite, or bleaching powder. Alternatively, it is obtained by passing chlorine into a hot aqueous suspension of lead sulfate and magnesium hydroxide. The ionic reaction is:
Pb(OH)3ˉ +ClOˉ → PbO2 + Clˉ+ OHˉ + H2O
It also is produced by electrolysis of acidic solutions of lead salts using a lead or platinum electrode. In such electrolytic process, lead dioxide is deposited on the anode of the cell.
Insoluble powdered lead dioxide also may be obtained when lead tetroxide is heated with nitric acid:
Pb3 O4 + 4HNO3 → 2Pb(N)3)2 + PbO2 + 2H2O
Lead dioxide also can be prepared by fusing lead monoxide with a mixture of sodium nitrate and sodium chlorate. |
| | LEAD HYDROXIDE Preparation Products And Raw materials |
|