|
|
| | 5α-Hydroxy-11-methoxy-1-methyl-9,10-(methylenebisoxy)lycorenan-7-one Basic information |
| Product Name: | 5α-Hydroxy-11-methoxy-1-methyl-9,10-(methylenebisoxy)lycorenan-7-one | | Synonyms: | 5α-Hydroxy-11-methoxy-1-methyl-9,10-(methylenebisoxy)lycorenan-7-one;Neronine;[1,3]Dioxolo[6,7][2]benzopyrano[3,4-g]indol-7(1H)-one, 2,3,5,5a,12b,12c-hexahydro-5-hydroxy-12-methoxy-1-methyl-, (5S,5aS,12bS,12cS)- | | CAS: | 1167-58-4 | | MF: | C18H19NO6 | | MW: | 345.34656 | | EINECS: | | | Product Categories: | | | Mol File: | 1167-58-4.mol | 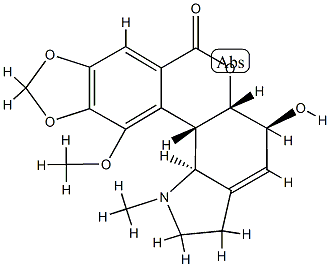 |
| | 5α-Hydroxy-11-methoxy-1-methyl-9,10-(methylenebisoxy)lycorenan-7-one Chemical Properties |
| Melting point | 196-7°C | | Boiling point | 558.7±50.0 °C(Predicted) | | density | 1.49±0.1 g/cm3(Predicted) | | pka | 12.97±0.20(Predicted) |
| | 5α-Hydroxy-11-methoxy-1-methyl-9,10-(methylenebisoxy)lycorenan-7-one Usage And Synthesis |
| Description | This alkaloid possesses a similar structure to that of Nerinine (q.v.) and is found
in Nerine krigeii. It may be crystallized from AcOEt and is dextrorotatory with
[0:]54 + 161.6° (c 0.57, CHC13). It is hygroscopic and behaves as a monoacidic
base yielding a picrate as yellow prisms, m.p. 205-9°C (dec.); a methiodide, m.p.
260-5°C (dec.) and a crystalline O-acetate, m.p. 20t-2°C. It contains a lactone
ring which may be opened by alkalies and regenerated by acids. | | References | Briggs et at., J. Arner. Chern. Soc., 78,2899 (1956)
Jeffs, Hawksworth., Tetrahedron Lett., 217 (1963) |
| | 5α-Hydroxy-11-methoxy-1-methyl-9,10-(methylenebisoxy)lycorenan-7-one Preparation Products And Raw materials |
|