- Atorvastatin
-

- $29.00 / 2mg
-
2024-10-28
- CAS:134523-00-5
- Min. Order:
- Purity: 99.69%
- Supply Ability: 10g
- Atorvastatin
-

- $0.00 / 1KG
-
2024-10-16
- CAS:134523-00-5
- Min. Order: 1KG
- Purity: 99%, USP
- Supply Ability: 5,000KG
- Atorvastatin
-

- $484.00 / 1KG
-
2024-08-30
- CAS:134523-00-5
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: 100KG
|
| | Atorvastatin Basic information |
| Product Name: | Atorvastatin | | Synonyms: | Atorvastatin&Ats-5Ats-8Ats-9M4L1;Atrovastitne;(bR,dR)-2-(p-Fluorophenyl)-β,δ-dihydroxy-5-isopropyl-3-phenyl-4-(phenylcarbamoyl)pyrrole-1-heptanoic acid;1H-Pyrrole-1-heptanoic acid, 2-(4-fluorophenyl)-β,δ-dihydroxy-5-(1-methylethyl)-3-phenyl-4-[(phenylamino)carbonyl]-, (βR,δR)-;1H-Pyrrole-1-heptanoic acid, 2-(4-fluorophenyl)-β,δ-dihydroxy-5-(1-methylethyl)-3-phenyl-4-[(phenylamino)carbonyl]-, [R-(R*,R*)]-;Cardyl;[R-(R^<*>^,R^<*>^)]-2-(4-Fluorophenyl)- β,δ-dihydroxy-5-(1-methylethyl)-3-phenyl-4-[(phenylarnino)carbonyl]-1H.Pyrrole-1- heptanoic acid;(3R,5R)-7-[2-(4-fluorophenyl)-5-isopropyl-3-phenyl-4-(phenylcarbamoyl)pyrrol-1-yl]-3,5-dihydroxy-enanthic acid | | CAS: | 134523-00-5 | | MF: | C33H35FN2O5 | | MW: | 558.65 | | EINECS: | 806-698-0 | | Product Categories: | | | Mol File: | 134523-00-5.mol | 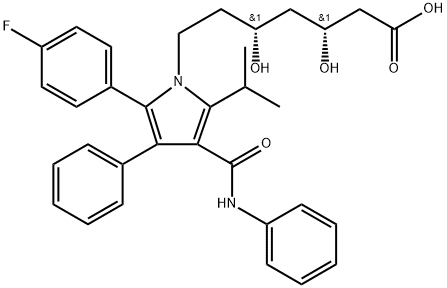 |
| | Atorvastatin Chemical Properties |
| | Atorvastatin Usage And Synthesis |
| Chemical Properties | white Crystalline powder | | Originator | Cardyl,Pfizer,Spain | | Uses | antihyperlipoproteimic;HMG-CoA reductase inhibition | | Definition | ChEBI: Atorvastatin is a dihydroxy monocarboxylic acid that is a member of the drug class known as statins, used primarily for lowering blood cholesterol and for preventing cardiovascular diseases. It has a role as an environmental contaminant and a xenobiotic. It is an aromatic amide, a member of monofluorobenzenes, a statin (synthetic), a dihydroxy monocarboxylic acid and a member of pyrroles. It is functionally related to a heptanoic acid. It is a conjugate acid of an atorvastatin(1-). | | Manufacturing Process | 285 ml 2.2 M n-butyl lithium in hexane was added dropwise to 92 ml
diisopropylamine at -50-60°C under nitrogen. The well stirred solutions
warmed to about -20°C, then it was cannulated into a suspension of 99 g of
S(+)-2-acetoxy-1,1,2-triphenylethanol in 500 ml absolute tetrahydrophuran
(THF) at -70°C and the reaction mixture was allowed to warm to -10°C for 2
hours. A suspension of MgBr2 was made from 564 ml (0.63 mol) of bromine
and 15.3 g of magnesium (0.63 mol) in 500 ml THF cooled to -78°C. The
enolate solution was cannulated into this suspension within 30 min and was
stirred for 60 min at -78°C. 150 g 5-(4-fluorophenyl)-2-(1-methylethyl)-1-(3-
oxopropyl)-N,4-diphenyl-1H-pyrrole-3-carboxamide in 800 ml absolute THF
was added dropwise over 30 min, stirred 90 min at -78°C, then was added
200 ml acetic acid, this is removed to a cool bath, 500 ml of H2O was added
and the mixture concentrate in vacuo at 40-50°C. After adding of 500 ml of
1:1 EtOAc/heptane the mixture was filtered. The filtrate was washed
extensively with 0.5 N HCl, then several times with H2O and finally EtOAc/heptane (3:1) and cooled with dry ice to -20°C. The light brown
crystalline product was dried in vacuum oven at 40°C. The yield was 194 g.
112 g of the same product was produced by evaporation of mother liquor
after recrystallization and chromatographic purification on a silicagel.
162 g of this substance was suspended in methanol/THF (5:3) and was stirred
with 11.7 g of sodium methoxide until everything was dissolved and kept in
the freezer overnight. Later it was quenched with AcOH concentrated in vacuo,
was added to 500 ml H2O and extracted twice with EtOAc (300 ml). The
combined extracts was washed with saturated NaHCO3 brine and dried over
anhydrous MgSO4, purified on silica-gel and gave 86.1 g of white crystals m.p.
125-126°C, αD
20=4.23° (1.17 M, CH3OH).
81 g of the last product in 500 ml absolute THF was added as quickly as
possible to the mixture of 77 ml THF at diisopropylamine, 200 ml 2.2 M of nbutyl
lithium and 62 ml of t-butylacetate in 200 ml THF -40-42°C under
nitrogen. Stirring was continued for 4 hours at -70°C. The reaction mixture
was concentrated in vacuo, the residue was taken up in EtOAc, washed with
water, then saturated NH4Cl, NaHCO3 (saturated), dried over anhydrous
MgSO4, filtred and the solvent evaporated.
The organic phase was dried and concentrated in vacuo to yield 73 g crude
product, that was dissolved in 500 ml absolute THF, 120 ml triehtylborane and
0.7 g t-butylcarboxylic acid, 70 ml methanol and 4.5 g sodium borohydride
was added. The mixture was stirred at -78°C under a dry atmosphere for 6
hours, poured slowly into 4:1:1 mixture of ice/30%H2O2/H2O and stirred
overnight. CHCl3 (400 ml) was added and organic layer washed extensively
with H2O until no peroxide could be found, was dried over MgSO4, filtered and
was treated by chromatography on silica gel to yield 51 g. The product was
dissolved in THF/methanol and saponificated with NaOH and, concentrated to
remove organic solvents at room temperature, added 100 ml H2O, and
extracted with Et2O twice. Organic layer was thoroughly dried and it was left
at room temperature for the next 10 days, then concentrated.
Chromatography on silica gel yielded 13.2 g racemate of lactone - trans-(+/-
)-5-(4-fluorophenyl)-2-(1-methylethyl)-N,4-di-diphenyl-1-[2-(tetrahydro-4-
hydroxy-6-oxo-2H-pyran-2-yl)ethyl]-1H-pyrrole-3-carboxamide. This racemate
was divided by chiral synthesis which was made analogously the method in US
Pat. No. 4,581,893. Then each isomer was saponificated with NaOH and
purificated by HPLC. The calcium salt corresponding acid was prepared by
reaction with 1 eq. of CaCl2·2H2O in water. | | Brand name | Lipitor
(Pfizer). | | Therapeutic Function | Anticholesteremic | | General Description | Atorvastatin, [R-(R*,R*)]-2-(4-fluorophenyl)-b,d-dihydroxy-5-(1-methylethyl)-3-phenyl-4-[(phenylamino)carbonyl]-lH-pyrrole-1-heptanoic acid(Lipitor), also possesses the heptanoic acid side chain,which is critical for inhibition of HMG-CoA reductase.Although the side chain is less lipophilic than the lactoneform, the high amount of lipophilic substitution causes this agent to have a slightly higher level of CNS penetration thanpravastatin, resulting in a slight increase in CNS side effects.Even so, its CNS profile is much lower than that of lovastatin.Atorvastatin is marketed as a combination therapywith amlodipine under the trade name Norvasc for managementof high cholesterol and high blood pressure. | | General Description | Cerivastatin (Baycol) is one of the neweragents in this class of cholesterol-lowering agents. However,it carries a higher incidence of rhabdomyolysis and, as a result,was voluntarily withdrawn from the market by its manufacturerin 2001. |
| | Atorvastatin Preparation Products And Raw materials |
|