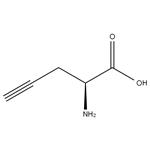
- L-Propargylglycine
-
- $0.00 / 1kg
-
2024-05-31
- CAS:23235-01-0
- Min. Order: 1kg
- Purity: 98%
- Supply Ability: 1T+
- L-Propargylglycine
-

- $14.00 / 1kg
-
2023-03-16
- CAS:23235-01-0
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 5000 tons
|
| | L-Propargylglycine Basic information |
| | L-Propargylglycine Chemical Properties |
| Boiling point | 272.1±35.0 °C(Predicted) | | density | 1.209±0.06 g/cm3(Predicted) | | storage temp. | Inert atmosphere,2-8°C | | pka | 2.04±0.10(Predicted) | | form | Powder | | color | White to pale yellow | | BRN | 2347861 | | InChIKey | DGYHPLMPMRKMPD-BYPYZUCNSA-N | | CAS DataBase Reference | 23235-01-0(CAS DataBase Reference) |
| Safety Statements | 22-24/25 | | WGK Germany | 3 | | F | 10 | | HS Code | 29224999 |
| | L-Propargylglycine Usage And Synthesis |
| Chemical Properties | White powder | | Definition | ChEBI: L-propargylglycine is a non-proteinogenic L-alpha-amino acid that is L-alanine in which one of the methyl hydrogens has been replaced by an ethynyl group. It causes the irreversible inactivation of gamma-cystathionase (also known as cystathionine gamma-lyase) and is used as an affinity labeling reagent for gamma-cystathionase and other enzymes. It has a role as an EC 1.4.3.2 (L-amino-acid oxidase) inhibitor, an EC 2.6.1.2 (alanine transaminase) inhibitor and an EC 2.5.1.48 (cystathionine gamma-synthase) inhibitor. It is a non-proteinogenic L-alpha-amino acid and a terminal acetylenic compound. It is a tautomer of a L-propargylglycine zwitterion. | | Enzyme inhibitor | This alkynyl amino acid and mechanism-based inhibitor (FW = 113.11 g/mol; CAS 23235-01-0), also known as (S)-2-amino-4-pentynoic acid, irreversibly inactivates γ-cystathionase (an enzyme that also catalyzes the synthesis of the metabolic signaling gas, H2S, See also Hydrogen Sulfide), acting as a Michael addition type suicide inhibitor of cystathionine γ-lyase, cystathionine γ-synthase, alanine aminotransferase, and methionine γ-lyase. Propargylglycine inactivates γ-cystathionase with pseudo-first-order kinetics and incorporation of 1 mol inhibitor per 80 kDa enzyme. When studied in vivo, inactivation of cystathionine γ-lyase in rat kidney was less than that in the liver, owing to the presence of a higher cysteine concentration in kidney. L-Propargylglycine was found to inactivate pig heart L-alanine transaminase (EC 2.6.1.2) at 37o C with a Ki = 3.9 mM, an observed maximal first order rate constant, kinact = 0.26 min–1, a minimal stoichiometric ratio necessary for inactivation of 2.7 L-propargylglycine molecules/enzyme subunit, with 2.2 molecules/subunit undergoing transamination before inactivation ensues. Experimental cystathioninuria, induced in rats by administration of D,L-propargylglycine, results in the formation of the cystathionine metabolites, cystathionine ketimine and perhydro-1,4-thiazepine-3,5-dicarboxylic acid, in various regions of the brain. L-Propargylglycine irreversibly inactivates proline dehydrogenase, which catalyzes the first step of proline catabolism, oxidizing proline to pyrroline-5-carboxylate. The 1.9-? resolution structure of the inactivated Thermus thermophilus enzyme shows that N5 of the flavin cofactor is covalently connected to the e-amino group of Lys-99 via a three-carbon linkage, consistent with the mass spectral analysis of the inactivated enzyme. The isoalloxazine ring has a butterfly angle of 25o, suggesting the cofactor is reduced. Two mechanisms, both involving oxidation to N-propargyliminoglycine, can account for these properties. L-Propargylglycine irreversibly inhibits L-amino acid oxidase from the venom of Crotalus adamanteus (Eastern diamondback rattlesnake) and Crotalus atrox (Western diamondback rattlesnake) in a dose- and timedependent manner that was blocked by the substrate L-phenylalanine. Other targets include methionineγ-lyase and UDP-Nacetylmuramate: L-alanine ligase, or L-alanine-adding enzyme, or UDP-Nacetylmuramoyl- L-alanine synthetase. | | Purification Methods | The acid crystallises readily when ~4g in 50mL H2O are treated with absolute EtOH at 4o/3hours, and is collected, washed with cold absolute EtOH and Et2O and dried in a vacuum. Also, it recrystallises from aqueous Me2CO, RF on SiO2 TLC plates with n-BuOH/H2O/AcOH (4:1:1) is 0.26. The racemate has m 238-240o. [Leukart et al. Helv Chim Acta 59 2181 1976, Eberle & Zeller Helv Chim Acta 68 1880 1985, Jansen et al. Recl Trav Chim Pays-Bas 88 819 1969.] It is a suicide inhibitor of �-cystathionase and other enzymes [Washtier & Abeles Biochemistry 16 2485 1977, Shinozuka et al. Eur J Biochem 124 377 1982]. |
| | L-Propargylglycine Preparation Products And Raw materials |
|