- Sotalol
-

- $0.00 / 100g
-
2023-11-03
- CAS:3930-20-9
- Min. Order: 1g
- Purity: 99%
- Supply Ability: 100000tons
- Sotalol
-

- $90.00/ kg
-
2023-10-20
- CAS:3930-20-9
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 10000/month
- Sotalol
-

- $90.00/ kg
-
2023-10-20
- CAS:3930-20-9
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 10000/month
|
| | Sotalol Basic information |
| Product Name: | Sotalol | | Synonyms: | SOTALOL;4'-[1-Hhydroxy-2-(isopropylamino)ethyl]methanesulfonanilide;4'-[1-Hydroxy-2-(isopropylamino)ethyl]metahnesulfonanilide;4'-[1-Hydroxy-2-(isopropylamino)ethyl]methanesulfonanilide;4’-(1-hydroxy-2-(isopropylamino)ethyl)-methanesulfonanilid;4’-(1-hydroxy-2-(isopropylamino)ethyl)methanesulfonanilide;beta-Cardone;dl-sotalol | | CAS: | 3930-20-9 | | MF: | C12H20N2O3S | | MW: | 272.36 | | EINECS: | | | Product Categories: | | | Mol File: | 3930-20-9.mol | 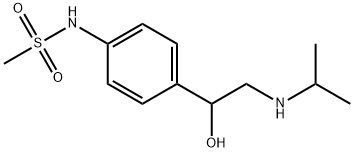 |
| | Sotalol Chemical Properties |
| Melting point | 206.5-207 °C | | Boiling point | 443.3±55.0 °C(Predicted) | | density | 1.298 g/cm3 | | storage temp. | Hygroscopic, -20°C Freezer, Under inert atmosphere | | solubility | Chloroform (Slightly, Heated), Methanol (Slightly, Sonicated) | | pka | pK1 8.2, pK2 9.8(at 25℃) | | form | Solid | | color | Pale Yellow to Light Yellow | | BCS Class | 1 | | Stability: | Hygroscopic | | InChI | InChI=1S/C12H20N2O3S/c1-9(2)13-8-12(15)10-4-6-11(7-5-10)14-18(3,16)17/h4-7,9,12-15H,8H2,1-3H3 | | InChIKey | ZBMZVLHSJCTVON-UHFFFAOYSA-N | | SMILES | CS(NC1=CC=C(C(O)CNC(C)C)C=C1)(=O)=O | | CAS DataBase Reference | 3930-20-9(CAS DataBase Reference) | | NIST Chemistry Reference | Methanesulfonanilide, 4'-(1-hydroxy-2-(isopropylamino)ethyl)-(3930-20-9) | | EPA Substance Registry System | Methanesulfonamide, N-[4-[1-hydroxy-2-[(1-methylethyl)amino]ethyl]phenyl]- (3930-20-9) |
| | Sotalol Usage And Synthesis |
| Originator | Sotagard,Glaxo | | Definition | ChEBI: A sulfonamide that is N-phenylmethanesulfonamide in which the phenyl group is sustituted at position 4 by a 1-hydroxy-2-(isopropylamino)ethyl group. It has both beta-adrenoreceptor blocking (Vaughan Williams Class II) and
ardiac action potential duration prolongation (Vaughan Williams Class III) antiarrhythmic properties. It is used (usually as the hydrochloride salt) for the management of ventricular and supraventricular arrhythmias. | | Manufacturing Process | As a resulting of reaction of methansulfonylchloride reacted with aniline
methansulfonanilide was obtained. The methansulfonanilide reacted with
bromacetylbromide at the presence of AlCl3 and CS2 and 4-(bromacetyl)-
methansulfonanilide was prepeared.
Then to the 4-(bromacetyl)-methansulfonanilide isopropylamine was added to
give 4-(1-oxo-2-isopropylaminoethyl)methansulfonanilide.The 4-(1-oxo-2-isopropylaminoethyl)methansulfonanilide was reduced by
hydrogenesation in the presence of Pd-C catalyst and sodium borohydride. So
4-(1-hydroxy-2-isopropylaminoethyl)methansulfonanilide was obtained.
The 4-(1-hydroxy-2-isopropylaminoethyl)methansulfonanilide hydrochloride
may be prepared by treatment of base with hydrochloric acid. | | Therapeutic Function | Beta-adrenergic blocker, Antiarrhythmic | | World Health Organization (WHO) | Sotalol is a non-selective?-adrenoreceptor antagonist. It should
be noted that when stopping sotalol the dose should be reduced gradually. | | General Description | Sotalol, 4'[1-hydroxy-2-(isopropylamino)ethyl]methylsulfonanilide (Betapace), is a relatively new antiarrhythmicdrug, characterized most often as a class IIIagent, and although it has effects that are related to the classII agents, it is not therapeutically considered a class II antiarrhythmic.It contains a chiral center and is marketed as theracemic mixture. Because of its enantiomers, its mechanismof action spans two of the antiarrhythmic drug classes. Thel(-) enantiomer has both β-blocking (class II) and potassiumchannel-blocking (class III) activities. The d(+) enantiomerhas class III properties similar to those of the (-) isomer,but its affinity for the -adrenergic receptor is 30 to 60 timeslower. | | Biological Activity | sotalol hydrochloride is an adrenergic beta-antagonist that is used in the treatment of life-threatening arrhythmias. | | Mechanism of action | Sotalol acts as an antiarrhythmic by β-
receptor blockade as well as by prolonging the
action potential duration in atrium and ventricle
and the refractory period in atrium and AV node
. Activity against supraventricular and ventricular
arrhythmiaswas demonstrated. | | Clinical Use | The sotalol enantiomers produce different effects onthe heart. Class III action of d-sotalol in the sinus node isassociated with slowing of sinus heart rate, whereas -adrenergicblockade contributes to the decrease in heart rate observedwith 1- or d,1-sotalol. Sotalol is not metabolized, noris it bound significantly to proteins. Elimination occurs byrenal excretion, with more than 80% of the drug eliminatedunchanged. Sotalol is characteristic of class III antiarrhythmicdrugs, in that it prolongs the duration of the action potentialand, thus, increases the effective refractory period of myocardialtissue. It is distinguished from the other class III drugs(amiodarone and bretylium) because of its -adrenergicreceptor–blocking action. | | Side effects | Side effects of sotalol include those attributed to both
β-adrenoceptor blockade and proarrhythmic effects.
This arrhythmia is a serious threat, as it may lead to ventricular
fibrillation. Adverse effects attributable to its β-
blocker activity include fatigue, dyspnea, chest pain,
headache, nausea, and vomiting. | | Drug interactions | Drugs with inherent QT interval–prolonging activity
(i.e., thiazide diuretics and terfenadine) may enhance
the class III effects of sotalol. | | Precautions | The contraindications that apply to other β-adrenoceptor
blocking agents also apply to sotalol. In addition, hypokalemia
and drugs known to prolong the QT interval
may be contraindicated, as they enhance the possibility
of proarrhythmic events. |
| | Sotalol Preparation Products And Raw materials |
|