- Dicloxacillin
-

- $1.00 / 1KG
-
2024-08-11
- CAS:3116-76-5
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: 20T
- Dicloxacillin
-
- $1.00 / 1KG
-
2020-02-01
- CAS:3116-76-5
- Min. Order: 1KG
- Purity: Min98% HPLC
- Supply Ability: G/KG/TON
|
| | Dicloxacillin Basic information |
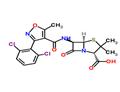
| Product Name: | Dicloxacillin | | Synonyms: | )-5-methyl-4-isoxazolyl)carbonyl)amino)-3,3///;4-thia-1-azabicyclo(3.2.0)heptane-2-carboxyl///;4-thia-1-azabicyclo(3.2.0)heptane-2-carboxylicacid,6-(((3-(2,6-dichlorophenyl;6-(3-(2,6-dichlorophenyl)-5-methyl-4-isoxazolecarboxamido)penicillanicacid[qr;6-dichlorophenyl)-5-methyl-4-isoxazolecarboxamido)-3,3-dimethyl-7-oxo-6-(3-(;brl1702;6-[3-(2,6-Dichlorophenyl)-5-methyl-4-isoxazolecarboxamido]-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylic acid;R 13423 | | CAS: | 3116-76-5 | | MF: | C19H17Cl2N3O5S | | MW: | 470.33 | | EINECS: | 221-488-3 | | Product Categories: | | | Mol File: | 3116-76-5.mol |  |
| | Dicloxacillin Chemical Properties |
| Melting point | 218 °C (decomp) | | Boiling point | 692.4±55.0 °C(Predicted) | | density | 1.62±0.1 g/cm3(Predicted) | | storage temp. | 2-8°C | | pka | pKa 2.76(H2O
t=37
I=0.15 (KCl)) (Uncertain) | | Water Solubility | 3.63 mg/L | | CAS DataBase Reference | 3116-76-5(CAS DataBase Reference) |
| | Dicloxacillin Usage And Synthesis |
| Description | Chemically this is 3(2,6-dichlorophenyl)-5-methyl-4-isoxazolyl penicillin.
It differs from cloxacillin by having two chloride ions attached to
the phenyl group. It comes as oral capsules of 250 and 500 mg, and in
an injectable formulation of 500 mg and 1 g. | | Originator | Dynapen,Bristol,US,1968 | | Uses | Antibacterial. | | Definition | ChEBI: A penicillin that is 6-aminopenicillanic acid in which one of the amino hydrogens is replaced by a 3-(2,6-dichlorophenyl)-5-methyl-1,2-oxazol-4-yl]formyl group. | | Manufacturing Process | A suspension of 6-aminopenicillanic acid (216 grams) in water (2 liters) was
adjusted to pH 6.8 by the addition of N aqueous sodium hydroxide
(approximately 1 liter) and the resulting solution was stirred vigorously while
a solution of 3-(2',6'-dichlorophenyl)-5-methylisoxazole-4-carbonyl chloride
(290 grams) in acetone (1.5 liters) was added in one portion.
The temperature rose to 26°C and as reaction proceeded the free acid form of
the penicillin separated as a white solid. After 30 minutes the suspension was
cooled to 10°C and stirring was continued at this temperature for 1 hour
more. The mixture was then cooled to 0°C, centrifuged, and the solid product
washed with aqueous acetone (250 ml) and finally dried in an air oven at
30°C. The product (440 grams, 94%) had [α]D20 +106.3° (c 1 in EtOH) and
was shown by alkalimetric assay to be 97.5% pure.
The salt was prepared by dissolving the free acid form of the penicillin in the
equivalent amount of aqueous sodium bicarbonate and freeze drying the
resulting solution. The hydrated salt so obtained was shown by alkalimetric
assay to be 94% pure and to contain 6% water. | | Therapeutic Function | Antibacterial | | Antimicrobial activity | It is very highly bound to serum
protein, and its activity in the presence of human serum
in vitro is depressed to a greater extent than that of other
isoxazolylpenicillins. | | General Description | Dicloxacillin was synthesized by Bayer in 1965 starting with 6-aminopenicillanic acid. It is a penicillinase-stable and orally active semisynthetic penicillin and shows higher and longer serum concentrations than cloxacillin when administered orally. Dicloxacillin is used orally, either alone or in combination with ampicillin, to treat various infections, including those caused by benzylpenicillin-resistant bacteria. | | Pharmacology | In terms of mechanism of action, antibacterial spectrum, and indications for use, it is
essentially no different than oxacillin and cloxacillin. Synonyms of this drug are diclocil,
novapen, diclex, and others.
The following type of semisynthetic penicillins that should be considered are those in
which amino acids, mainly α-aminophenylacetic or p-oxy-α-amino-phenylacetic acids,
act as the acyl radical (ampicillin, amoxacillin).
The antimicrobial spectrum of aminopenicillins is similar to penicillin G, with the
exception that they also act on a number of Gram-negative microorganisms. Both
aminopenicillins are destroyed by staphylococcus penicillinase. | | Pharmacokinetics | Oral absorption: c. 50%
Cmax , 250 mg oral: 9 mg/L after 1 h
500 mg intramuscular: 14–16 mg/L after 0.5–1 h
Plasma half-life: 0.5 h
Plasma protein binding: 95–97%
Absorption
Absorption in the very young is poor and unpredictable.
Metabolism and excretion
Dicloxacillin is partly metabolized in the liver and about
10% of the circulating drug is in the form of metabolites.
Some 50–70% of a dose is excreted in the urine, about
20% as metabolites. It is eliminated both in the glomerular
filtrate and by tubular secretion, and plasma concentrations
are raised by probenecid. Parent drug and increased
proportions of metabolites accumulate in renal failure.
Elimination is increased through enhanced tubular secretion
in patients with cystic fibrosis. | | Side effects | Phlebitis is common after intravenous injection. Its toxicity
is otherwise similar to that of other penicillins.
Clinical uses are those of the group 3 penicillins. |
| | Dicloxacillin Preparation Products And Raw materials |
|