- Potassium Citrate
-

- $10.00 / 1kg
-
2024-10-31
- CAS:866-84-2
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 20ton
- Potassium Citrate
-

- $900.00/ ton
-
2024-09-11
- CAS:866-84-2
- Min. Order: 3ton
- Purity: 99%
- Supply Ability: 500 tons
- Potassium Citrate
-

- $100.00 / 1kg
-
2024-07-01
- CAS:866-84-2
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 1t
|
| Product Name: | Potassium Citrate | | Synonyms: | tripotassium 2-hydroxypropane-1,2,3-tricarboxylate;2-Hydroxy-1,2,3-propanetricarboxylic acid tripotassium salt;Tripotassium-2-hydroxypropane-1,2,3-tricarboxylateanhydrous;Tripotassiumcitrate:forfood,E332;1,2,3-Propanetricarboxylicacid,2-hydroxy-,tripotassiumsalt;2,3-propanetricarboxylicacid,2-hydroxy-tripotassiumsalt;kajos;potassiuM 2-hydroxypropane-1,2,3-tricarboxylate | | CAS: | 866-84-2 | | MF: | C6H9KO7 | | MW: | 232.23 | | EINECS: | 212-755-5 | | Product Categories: | Buffer SolutionsBiochemicals and Reagents;ChelatorsProtein Structural Analysis;AlphabeticalBiological Buffers;Biological Buffers;BioUltra Buffers;Optimization Reagents;X-Ray Crystallography;Food additives;866-84-2 | | Mol File: | 866-84-2.mol | 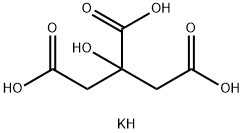 |
| | Potassium Citrate Chemical Properties |
| Melting point | decomposes at 230℃ [KIR78] | | density | 1.187 | | solubility | H2O: 1 M at 20 °C, clear, colorless | | PH | 8.59(1 mM solution);8.9(10 mM solution);9.01(100 mM solution);7.47(1000 mM solution) | | Odor | at 100.00?%. odorless | | Water Solubility | 60.91 g/100g saturated solution in water (25°C) [MER06] | | λmax | λ: 260 nm Amax: 0.045
λ: 280 nm Amax: 0.025 | | InChI | InChI=1S/C6H8O7.K.H/c7-3(8)1-6(13,5(11)12)2-4(9)10;;/h13H,1-2H2,(H,7,8)(H,9,10)(H,11,12);; | | InChIKey | JOSYFFAWJFRGHM-UHFFFAOYSA-N | | SMILES | C(O)(C(=O)O)(CC(=O)O)CC(=O)O.[KH] | | LogP | -0.280 (est) | | CAS DataBase Reference | 866-84-2(CAS DataBase Reference) | | EPA Substance Registry System | 1,2,3-Propanetricarboxylic acid, 2-hydroxy-, tripotassium salt (866-84-2) |
| | Potassium Citrate Usage And Synthesis |
| Potassium citrate | The applications of potassium citrate (or tripotassium citrate) mainly derive from its alkaline properties. It is used to treat kidney stones composed of uric acid and cysteine, as well as other renal failures caused by excess acidity, including in veterinary applications. As an additive to soft drinks and other edibles, it also functions as an acid buffer, less bitter than other potassium salts, and with less sodium than trisodium citrate.
An additional use of potassium citrate is to supplement potassium in patients with hypokalemia, preventing and treating gout and arrhythmia.
| | Prevention of urinary calculi drug | Potassium citrate is the drug which is most commonly used to prevent calculus. It was found that in the urine pH value and citric acid value and have a very close relationship with the formation urinary tract calculus. In an acidic urine (pH <5.5), the uric acid solubility is very low, so it is likely to cause uric acid calculus; while in alkaline urine (pH> 7), it is easy to form calcium or magnesium phosphate calculus. 63% of urinary calculus patients are below normal urine citrate, and potassium citrate can provide a lot of citric acid to enhance the urine pH value. So for uric acid calculus, It has a very significant therapeutic effect to low calcium citrate calculus disease and tubular poisoning calcium calculus disease. But It will raise the concentration of potassium and lower the concentration of ammonium ion in urinary, which is because not only complements the citric acid, but also supplements of potassium. Since potassium metabolism is very quickly in the body, there is no change in blood potassium concentration. It has little effect on the body. The daily amount of total urine, oxalate, phosphate, sodium, magnesium, sulfate and uric acid and other aspects test from patients with uric acid calculus, it is found that it is not affected by taking potassium citrate.
| | Chemical properties | Colorless crystals or white crystalline powder, odorless, cool and salty, slight deliquescence, soluble in water and glycerin, insoluble in ethanol. Tests show that the product had no significant harm to the human body, ADI doesn’t need special provision (FAO/WHO, 1994).
| | Uses | As a preservative, stabilizing and pH buffering agents and others. China provides for various types of food, according to production needs to use appropriately.
Used in the food, pharmaceutical and other industries, as buffering agents, chelating agents, flavoring agents, stabilizers, antioxidants, emulsifying salts.
| | Production methods | Citric acid and potassium hydroxide or potassium bicarbonate derived products.
C6H8O7 + 3KHCO3 → CaH5K3O7 · H2O + 3CO2 ↑ + 2H2O
| | Reference | https://en.wikipedia.org/wiki/Potassium_citrate
https://kidneystones.uchicago.edu/how-citrate-gets-into-the-urine/
https://www.drugbank.ca/drugs/DB09125
http://www.urocit-k.com/
http://www.jungbunzlauer.com/en/products/special-salts/tripotassium-citrate.html
https://www.quora.com/Why-is-potassium-citrate-buffer-is-an-acid-buffer
| | Description | Potassium citrate is a potassium salt of citric acid with the molecular formula C6H5K3O7. It is a white, slightly hygroscopic crystalline powder. It is odorless with a saline taste.
As a food additive, potassium citrate is used to regulate acidity and is known as E number E332. Medicinally, it may be used to control kidney stones derived from either uric acid or cystine. | | Chemical Properties | Colorless or white crystals or powder;
cooling, saline taste; odorless; deliquescent. Soluble
in water and glycerol; almost insoluble in alcohol. | | Uses | For use as an electrolyte replenisher and in the treatment of hypokalemia. | | Uses | Potassium citrate is rapidly absorbed when given by mouth and is excreted in the urine. Since it is an alkaline salt it is effective in reducing the pain and frequency of urination when these are caused by highly acidic urine . It is used for this purpose in dogs and cats, but is chiefly employed as a non-irritating diuretic.
Potassium citrate is an effective way to treat/manage gout and arrhythmia, if the patient is hypokalemic.
It is widely used to treat urinary calculi (kidney stones) and is often used by patients with cystinuria . | | Uses | potassium citrate is it acts as a buffer, maintaining product pH constant, a pH adjuster and a product stabilizer. This is a potassium salt. | | Definition | ChEBI: The anhydrous form of the tripotassium salt of citric acid. | | Production Methods | Potassium citrate is produced by adding potassium bicarbonate or potassium carbonate to a solution of citric acid until effervescence ceases, filtering the solution and evaporating to granulation. | | Flammability and Explosibility | Non flammable | | Industrial uses | Potassium citrate is used in the United Kingdom as an over-the-counter drug for the relief from discomfort
experienced in mild urinary-tract infections by increasing the urinary pH. It should be not given to men if
they experience pain in the kidney area (risk of kidney stones) or if blood or pus is present in the urine. Also,
patients with raised blood pressure or diabetes should avoid taking potassium citrate without consultation
with their general practitioner (GP). Caution is generally advised to patients with renal impairment, cardiac
problems and the elderly [3a]. | | Safety Profile | Poison by intravenous
route. When heated to decomposition it
emits toxic fumes of K2O. | | Precautions | Potassium citrate is usually administered by mouth in dilute aqueous solution. This is because of its somewhat caustic effect on the stomach lining, and the potential for other mild health hazards.
In states where non-prescription potassium citrate is legal, the maximum allowable over-the-counter (OTC) dose for elemental potassium is regulated by the FDA to be no more than 100 mg (approximately 3 % of the daily allowance ) . Pure potassium citrate contains 38.28 % potassium. |
| | Potassium Citrate Preparation Products And Raw materials |
|