| Company Name: |
J & K SCIENTIFIC LTD.
|
| Tel: |
010-82848833 400-666-7788 |
| Email: |
jkinfo@jkchemical.com |
| Products Intro: |
Product Name:IsoxicaM
CAS:34552-84-6
Package:10g,1g
|
| Company Name: |
Spectrum Chemical Manufacturing Corp.
|
| Tel: |
021-021-021-67601398-809-809-809 15221380277 |
| Email: |
marketing_china@spectrumchemical.com |
| Products Intro: |
Product Name:IsoxicaM
CAS:34552-84-6
Package:1GM Remarks:I3250
|
|
| | ISOXICAM Basic information |
| Product Name: | ISOXICAM | | Synonyms: | )-,1,1-dioxide;2h-1,2-benzothiazine-3-carboxamide,4-hydroxy-2-methyl-n-(5-methyl-3-isoxazolyl;pacyl(antiinflammatory);ISOXICAM;4-Hydroxy-2-methyl-N-(5-methyl-3-isoxazolyl)-2H-1,2-benzothiazine-3-carboxamide-1,1-dioxide;Pacyl;Vectren;W 8495 | | CAS: | 34552-84-6 | | MF: | C14H13N3O5S | | MW: | 335.34 | | EINECS: | 252-084-5 | | Product Categories: | OCTOPIROX | | Mol File: | 34552-84-6.mol |  |
| | ISOXICAM Chemical Properties |
| Melting point | 265-271℃ | | density | 1.588 | | storage temp. | -20°C Freezer | | solubility | Chloroform (Slightly, Heated), DMSO (Slightly), Methanol (Slightly, Heated, Sonicated) | | pka | 4.50±1.00(Predicted) | | color | White to Pale Beige |
| WGK Germany | 2 | | RTECS | DL0703000 | | Toxicity | LD50 in rats (mg/kg): >5000 orally (Whitehouse) |
| | ISOXICAM Usage And Synthesis |
| Description | Isoxicam is a non-steroidal antiinflammatory agent useful in the treatment of
various forms of rheumatoid arthritis, osteoarthritis and musculoskeletal disorders.
It is about one-tenth as potent as its structural relative sudoxicam;
its similar long T1/2 (>30 hrs.) allows once-daily dosing. | | Originator | Warner-Lambert (USA) | | Uses | antiseborrheic | | Uses | Isoxicam is a potent, orally active, nonsteroidal anti-inflammatory drug. | | Uses | Isoxicam is used for the same indications as piroxicam. Synonyms for isoxicam are floxicam and
maxicam. | | Definition | ChEBI: A monocarboxylic acid amide that is piroxicam in which the pyrid-2-yl group is replaced by a 5-methyl-1,2-oxazol-3-yl group. A non-steroidal anti-inflammatory drug, it was withdrawn from the market in the 1980s following its association with cases of Steve
s-Johnson syndrome. | | Manufacturing Process | A mixture of 40.5 g (0.15 mol) of 3-carbethoxy-4-hydroxy-2-methyl-2H-1,2-
benzothiazine 1,1-dioxide, 20.6 g (0.21 mol) of 3-amino-5-methylisoxazole,
and 2,500 ml of xylene was refluxed for 24 hours in a Soxhlet apparatus, the
thimble of which contained 60 g of Linde type 4A molecular sieve. The mixture
was cooled to 25°C and the resulting crystalline precipitate was collected and
washed with ether to give 44 g of crude product. Recrystallization from 1,600
ml of 1,4-dioxan gave 34.7 g of material, MP 265°C to 271°C dec. | | Brand name | Maxicam (Parke-Davis);Floxicam;PACYL. | | Therapeutic Function | Antiinflammatory | | World Health Organization (WHO) | Isoxicam, a nonsteroidal anti-inflammatory agent, was introduced
in 1983 for the treatment of rheumatic disorders. By 1985 its use had been
associated with serious adverse effects, including four deaths from rare skin
reactions. This led to its withdrawal in France followed immediately by the
voluntary suspension of marketing worldwide by the major manufacturer. | | Clinical Use | Isoxicam is a nonsteroidal
anti-inflammatory drug withdrawn from the
market as a consequence of reports of fatal skin
reaction. | | Synthesis | Isoxicam, 1,1-dioxide 4-hydroxy-2-methyl-N-(5-methyl-3-isoxazolyl)-2H-1,2-
benzothiazine-3-carboxamide (3.2.80), is synthesized analogous to piroxicam, using ami�dation of 1,1-dioxide 3-methoxycarbonyl-3,4-dihydro-2-H-1,2-benzothiazine-4-one
(3.2.78) in the last stage with 3-amino-5-methylisoxazole, instead of 2-aminopyridine
[127¨C130]. 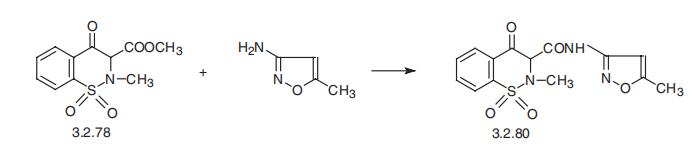
|
| | ISOXICAM Preparation Products And Raw materials |
|