| Company Name: |
Prolife Bio Chemical Industries Pvt Ltd
|
| Tel: |
+91-9825114995 +91-9825114995 |
| Email: |
jolly@prolifebiochem.com |
| Products Intro: |
Product Name:4-Amino-1,5-naphthalenedisulfonic acid
Purity:99% Package:1 kg,5 kg, 10 kg,25kg
|
| Company Name: |
Shenzhen SUNGENING Bio-Medical Co., Ltd.
|
| Tel: |
0755-28967200 13631290199 |
| Email: |
wxf@sungening.com |
| Products Intro: |
Product Name:Amino-S Acid
CAS:117-55-5
Purity:99% HPLC or More Package:10MG;25MG;50MG;100MG
|
|
| | 4-aminonaphthalene-1,5-disulphonic acid Basic information |
| Product Name: | 4-aminonaphthalene-1,5-disulphonic acid | | Synonyms: | 4-aminonaphthalene-1,5-disulphonic acid;4-Amino-1,5-naphthalenedisulfonic acid;8-Amino-1,5-naphthalenedisulfonic acid;Amino-S acid;1,5-Naphthalenedisulfonic acid, 4-amino- | | CAS: | 117-55-5 | | MF: | C10H9NO6S2 | | MW: | 303.31 | | EINECS: | 204-196-0 | | Product Categories: | | | Mol File: | 117-55-5.mol | 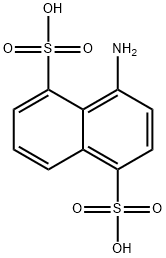 |
| | 4-aminonaphthalene-1,5-disulphonic acid Chemical Properties |
| Boiling point | 205.8°C (rough estimate) | | density | 1.6329 (rough estimate) | | refractive index | 1.7330 (estimate) |
| Toxicity | LD50 orl-rat: 56 g/kg GISAAA 45(3),73,80 |
| | 4-aminonaphthalene-1,5-disulphonic acid Usage And Synthesis |
| Chemical Properties | 1-Aminonaphthalene-4,8-disulfonic acid [117-55-5]. (4-aminonaphthalene-1,5- disulfonic acid), C10H9NO6S2, Mr 303.3, and its acid monosodium salt are sparingly soluble in cold water, but the disodium salt is readily soluble. Hydrolysis with sodium hydroxide at 200 ℃ yields 1-amino-8-hydroxynaphthalene-4-sulfonic acid; at higher temperature, 1,8-dihydroxynaphthalene-4-sulfonic acid is formed. Heating with bisulfite produces 1-hydroxynaphthalene4,8-disulfonic acid. Diazo compounds couple with 1-aminonaphthalene-4,8-disulfonic acid to form azo compounds, in contrast to 2-aminonaphthalene-4,8-disulfonic acid, which reacts to form diazoamino compounds. | | Production Methods | Naphthalene is sulfonated under conditions that give predominantly the 1,5- disulfonic acid, and the sulfonation mass is nitrated at 20 ℃. 3-Nitronaphthalene-1,5-disulfonic acid is precipitated from the quenched reaction mixture as its iron(II) salt and removed. After dilution and liming out, the remainder of the mixed nitro solution is reduced with iron and dilute acid, and the 1,4,8-isomer is salted out preferentially in ca. 40 % yield. A small proportion (ca. 10 % of 1-aminonaphthalene-3,8-disulfonic acid) is then salted out, after which the more soluble 1-aminonaphthalene-5,7-disulfonic acid crystallizes on concentration. | | Safety Profile | When heated to decomposition itemits toxic fumes of SOx and NOx. |
| | 4-aminonaphthalene-1,5-disulphonic acid Preparation Products And Raw materials |
|