|
|
| | Chlortetracycline hydrochloride Basic information |
| Product Name: | Chlortetracycline hydrochloride | | Synonyms: | 7-CHLORTETRACYCLINE MONOHYDROCHLORIDE;7-CHLOROTETRACYCLINE HCL;7-CHLOROTETRACYCLINE HYDROCHLORIDE;7-CHLOROTETRACYCLINE MONOHYDROCHLORIDE;2-Naphthacenecarboxamide, 7-chloro-4-(dimethylamino)-1,4,4a,5,5a,6,11,12a-octahydro-3,6,10,12,12a-pentahydroxy-6-methyl-1,11-dioxo-, monohydrochloride, [4S-(4alpha,4aalpha,5aalpha,6beta,12aalpha)]-;CHLOROTETRACYCLINE HYDROCHLORIDEVETRANAL , 250 MG;CHLORTETRACYCLINE HYDROCHLORIDE FROM STR EPTOMYCES AUREOFACI;Aureociclina, Isphamycin | | CAS: | 64-72-2 | | MF: | C22H24Cl2N2O8 | | MW: | 515.34 | | EINECS: | 200-591-7 | | Product Categories: | AUREOMYCIN;Intermediates & Fine Chemicals;Pharmaceuticals;Inhibitors;64-72-2 | | Mol File: | 64-72-2.mol | 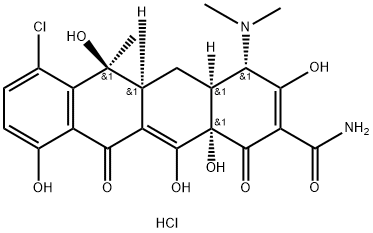 |
| | Chlortetracycline hydrochloride Chemical Properties |
| Melting point | 210-215 °C (dec.)(lit.) | | alpha | D23 -240° | | storage temp. | Inert atmosphere,Store in freezer, under -20°C | | solubility | 1 M NaOH: soluble50mg/mL | | form | Powder | | color | faint yellow to yellow | | pka | 3.3(at 25℃) | | Water Solubility | Soluble in water | | Sensitive | Light Sensitive | | Merck | 13,2211 | | BRN | 3858364 | | CAS DataBase Reference | 64-72-2(CAS DataBase Reference) | | EPA Substance Registry System | Chlortetracycline hydrochloride (64-72-2) |
| Hazard Codes | Xi | | Risk Statements | 36/37/38 | | Safety Statements | 26-36 | | WGK Germany | 2 | | RTECS | QI7800000 | | HazardClass | 3 | | HS Code | 29413020 | | Toxicity | LD50 orally in rats: 10300 mg/kg (Goldenthal) |
| Provider | Language |
|
[4S-(4alpha,4aalpha,5aalpha,6beta,12aalpha)]-7-Chloro-4-(dimethylamino)-1,4,4a,5,5a,6,11,12a-octahydro-3,6,10,12,12a-pentahydroxy-6-methyl-1,11-dioxo-2-naphthacenecarboxamide monohydrochloride
| English |
|
SigmaAldrich
| English |
|
ACROS
| English |
| | Chlortetracycline hydrochloride Usage And Synthesis |
| Description | Chlortetracycline was found in the culture broth of Streptomyces aureofaciens by Duggar et al. of Lederle Laboratories in 1948 and named aureomycin. It shows a wider range of antibiotic activity than the earlier antibiotics, penicillins, and streptomycins and as great as that of chloramphenicol. Its activity covers grampositive and gram-negative bacteria as well as Rickettsia and Chlamydiae. Chlortetracycline has been replaced by other tetracyclines in clinical use and is used now used as a feed additive to promote the growth of livestock. | | Chemical Properties | Yellow Solid | | Uses | Chlortetracycline hydrochloride is a salt prepared from chlortetracycline taking advantage of the basic dimethylamino group which protonates and readily forms the salt in hydrochloric acid solutions. The hydrochloride is the preferred formulation for pharmaceutical applications. Like all tetracyclines, chlortetracycline shows broad spectrum antibacterial and antiprotozoan activity and acts by binding to the 30S and 50S ribosomal sub-units, blocking protein synthesis. | | Uses | Chlortetracycline is an antibiotic substance isolated from the substrate of Streptomyces aureofaciens. Chlortetracycline is an antimicrobial and antibacterial. | | Uses | antibacterial, antiamebic, Ca chelator, hepatotoxic; inhibits protein synthesis | | Uses | An antimicrobial and antibacterial.
This compound contains an undetermined amount of epi-chlortetracycline | | Brand name | Aureomycin (Lederle). | | General Description | Chlortetracycline (Aureomycin hydrochloride) was isolatedby Duggar in 1948 from S. aureofaciens. This compound,which was produced in an extensive search for new antibiotics,was the first of the group of highly successful tetracyclines.It soon became established as a valuable antibioticwith broad-spectrum activities.
It is used in medicine chiefly as the acid salt of the compoundwhose systematic chemical designation is 7-chloro-4-(dimethylamino)-1,4,4a,5,5a,6,11,12a-octahydro-3,6,10,12,12a-pentahydroxy-6-methyl-1,11-dioxo-2-naphthacenecarboxamide.The hydrochloride salt is a crystallinepowder with a bright yellow color, which suggested itsbrand name, Aureomycin. It is stable in air but slightly photosensitiveand should be protected from light. It is odorlessand bitter. One gram of the hydrochloride salt will dissolvein about 75 mL of water, producing a pH of about 3. It isonly slightly soluble in alcohol and practically insoluble inother organic solvents.
Oral and parenteral forms of chlortetracycline are nolonger used because of the poor bioavailability and inferiorpharmacokinetic properties of the drug. It is still marketed inointment forms for topical and ophthalmic use. | | Purification Methods | Purify the salt by dissolving 1g rapidly in 20mL of hot water, cooling rapidly to 40o, treating with 0.1mL of 2M HCl, and chilling in an ice-bath. The process is repeated twice. It is also recrystallised from Me2NCHO/Me2CO. [Stephens et al. J Am Chem Soc 76 3568 1954, UV: McCormick et al. J Am Chem Soc 79 2849 1975, Beilstein 14 IV 2631.] |
| | Chlortetracycline hydrochloride Preparation Products And Raw materials |
|